A kind of preparation method containing rifampicin compound preparation
A compound preparation and rifampicin technology are applied in the field of preparation of compound preparations containing rifampicin, which can solve the problem that the control of the thickness of the granule coating layer is difficult to monitor and control, increases the production burden on production equipment and sites, and is difficult to ensure uniform content of the preparation. To solve problems such as sexuality, to achieve the effect of being conducive to industrialized large-scale production, shortening the production cycle, and ensuring the efficacy of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 different Fu tablets
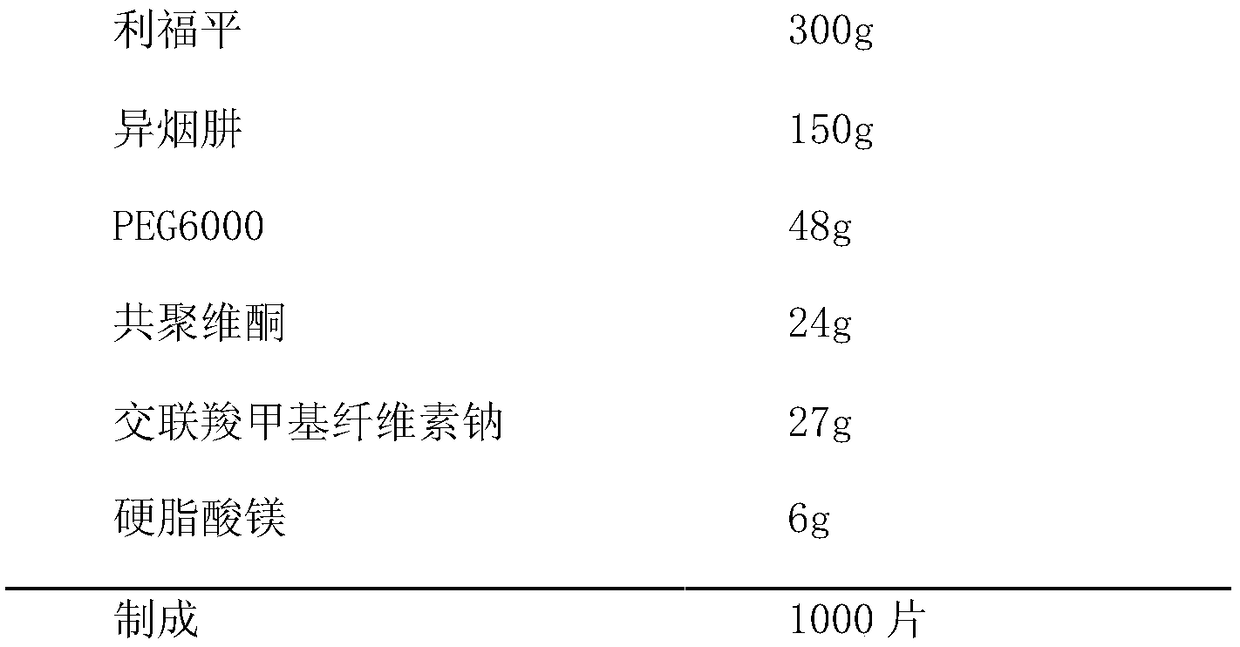
[0031] 1. Prescription
[0032] (1) plain tablet prescription
[0033]
[0034] (2) Film coating prescription
[0035] Hypromellose 20g
[0036] 95% ethanol 240g
[0037] Purified water 25g
[0038] 2. Preparation method
[0039] (1) Granulation: pass rifampicin, isoniazid, and PEG6000 through 80-mesh sieve and mix well, heat the mixture to 80-100°C, put it into a swing granulator and pass through a 16-mesh sieve to make granules, and cool to room temperature After 40 mesh sieve granulation.
[0040] (2) Tablet compression: Add the remaining materials in the plain tablet prescription, mix well, and press the granules into tablets with a Φ11mm round punch.
[0041] (3) Coating: Coating with a slurry prepared from hypromellose, and controlling the tablet bed temperature to not be higher than 50°C.
Embodiment 2
[0042] Embodiment 2 Different Fu Capsules
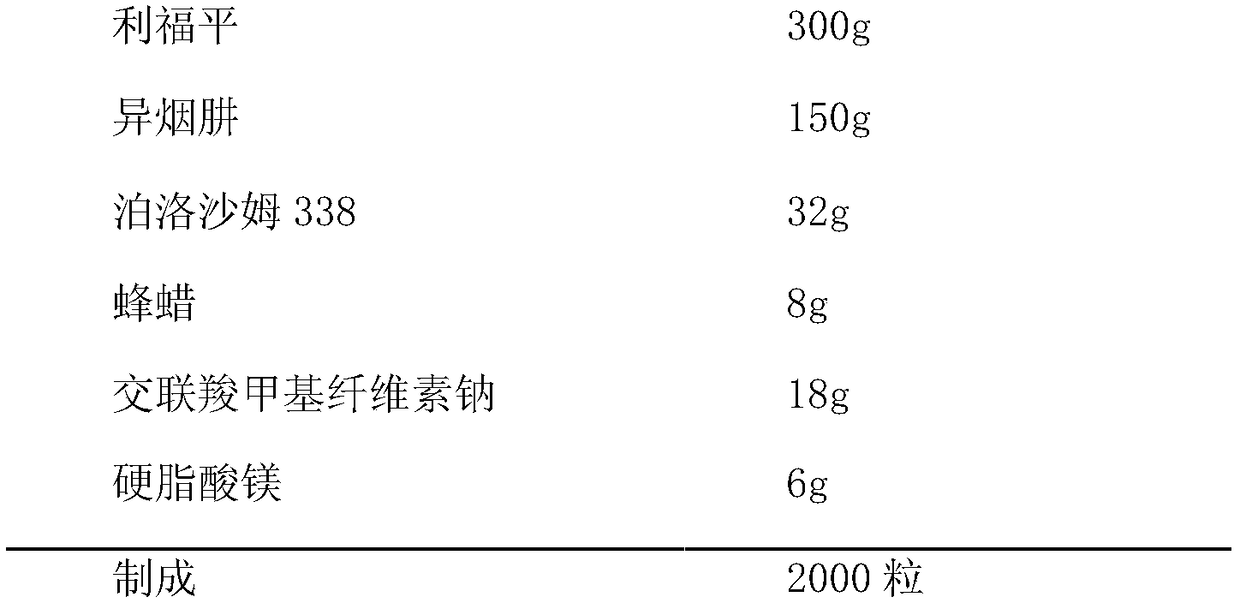
[0043] 1. Prescription
[0044]
[0045] 2. Preparation method
[0046] (1) Granulation: pass rifampicin, isoniazid, poloxamer 338, beeswax, and croscarmellose sodium through an 80-mesh sieve and mix well, and the mixture is granulated in a fluidized bed, cooled to After room temperature, 24 mesh sieve for granulation.
[0047] (2) Filling: Add magnesium stearate, mix well, and pack in 2000 No. 1 capsules.
Embodiment 3
[0048] Example 3 Isofamide Tablets
[0049] 1. Prescription
[0050] (1) plain tablet prescription
[0051]
[0052]
[0053] (2) Film coating prescription
[0054] Hypromellose 20g
[0055] 95% ethanol 240g
[0056] Purified water 25g
[0057] 2. Preparation method
[0058] (1) Granulation: Pass rifampicin, isoniazid, pyrazinamide, PEG6000, poloxamer 188, and carnauba wax through 80-mesh sieves and mix well, heat the mixture to 80-120°C, add and shake Granulator, 16-mesh sieve to make granules, after cooling to room temperature, 40-mesh sieve for granulation.
[0059] (2) Tablet compression: Add the remaining materials in the plain tablet prescription, mix well, and press the granules into tablets with a Φ11mm round punch.
[0060] (3) Coating: Coating with a slurry prepared from hypromellose, and controlling the tablet bed temperature to not be higher than 50°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com