Compound tablet of lamivudine, zidovudine and efavirenz and preparation method thereof
A technology of zidovudine and lamivudine, which is applied in the field of compound tablets of lamivudine, zidovudine and efavirenz and its preparation, can solve the problem of reduced drug efficacy, no compound tablets, effective Composition loss and other issues to achieve the effect of reducing drug resistance, reducing mutual contact, and reducing degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0029] Experimental Example 1: Grind sodium chloride to below 70 μm, weigh 15g of lamivudine, 1.51g of ground sodium chloride, 0.3g of croscarmellose sodium and feed them into a fluidized bed granulator 10% starch slurry is sprayed in at a constant speed as a binder to fully mix the binder and the weighed material, and lamivudine pellets are prepared in a fluidized bed granulator. Then send it into the coating equipment, and control the temperature of the coating equipment at 45-50° C. to prepare the hydroxypropyl methylcellulose coating liquid, and prepare the lamivudine coated pellets by spraying the coating liquid.
experiment example 2
[0030]Experimental Example 2: Mix sodium chloride and water in a weight ratio of 1:5 and stir until dissolved, then put it into an atomizer, and obtain sodium chloride powder by spray drying. Spray drying is a prior art and will not be described in detail here. Weigh 15g of lamivudine, 1.51g of spray-dried sodium chloride powder, 0.3g of croscarmellose sodium and feed them into a fluidized bed granulator, using 10% starch slurry as a binder Spray at a uniform speed to fully mix the binder and the weighed material, and prepare lamivudine pellets in a fluidized bed granulator. Then send it into the coating equipment, and control the temperature of the coating equipment at 45-50° C. to prepare the hydroxypropyl methylcellulose coating liquid, and prepare the lamivudine coated pellets by spraying the coating liquid.
[0031] Five batches of samples were prepared according to the methods of Experimental Example 1 and Experimental Example 2, respectively, and the content of lamivud...
Embodiment 1
[0036] (1) Preparation of lamivudine coated pellets
[0037]
[0038] The lamivudine, sodium chloride and croscarmellose sodium of prescription quantity are weighed and fed into the fluidized bed granulator, and 10% starch slurry is sprayed in at a uniform speed as a binding agent to make the binding agent The agent and the weighed material are fully mixed, and the lamivudine pellets are prepared in a fluidized bed granulator. Then send it into the coating equipment, and control the temperature of the coating equipment at 45-50° C. to prepare the hydroxypropyl methylcellulose coating liquid, and prepare the lamivudine coated pellets by spraying the coating liquid.
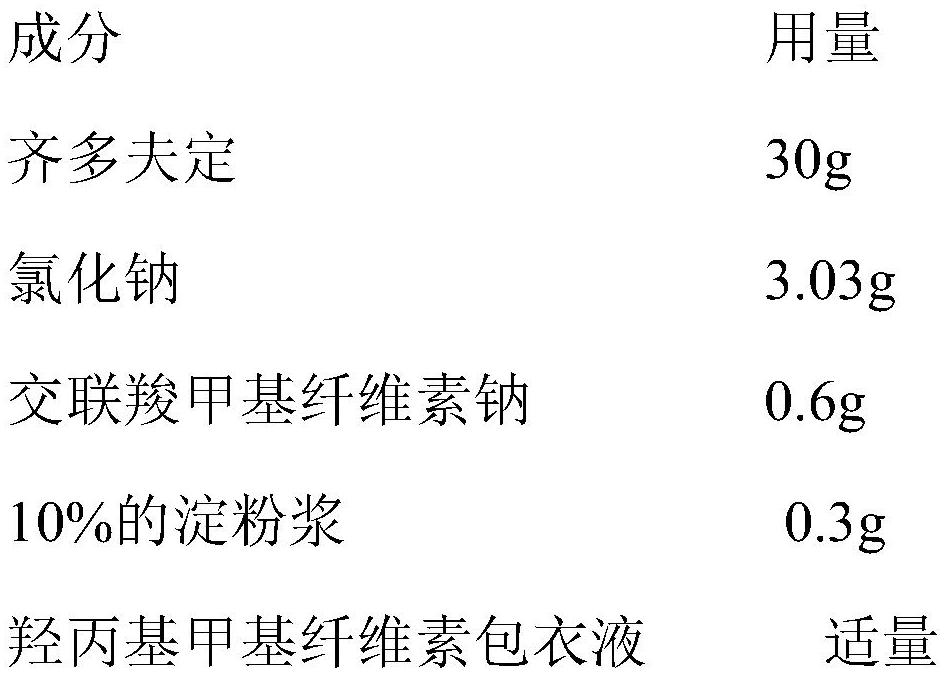
[0039] (2) Preparation of zidovudine coated pellets
[0040]
[0041] Zidovudine, sodium chloride and croscarmellose sodium of prescription quantity are weighed and fed into the fluidized bed granulator, and 10% starch slurry is sprayed in at a uniform speed as a binding agent to make the binding agent The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com