O-carborane phenol derivative as well as preparation method and application thereof
A technology of phenol derivatives and o-carborane, which is applied in the field of anti-tumor compounds, can solve the problems of limited application and low accumulation concentration of tumor tissues, and achieve the effects of reasonable route, easy control of reaction and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
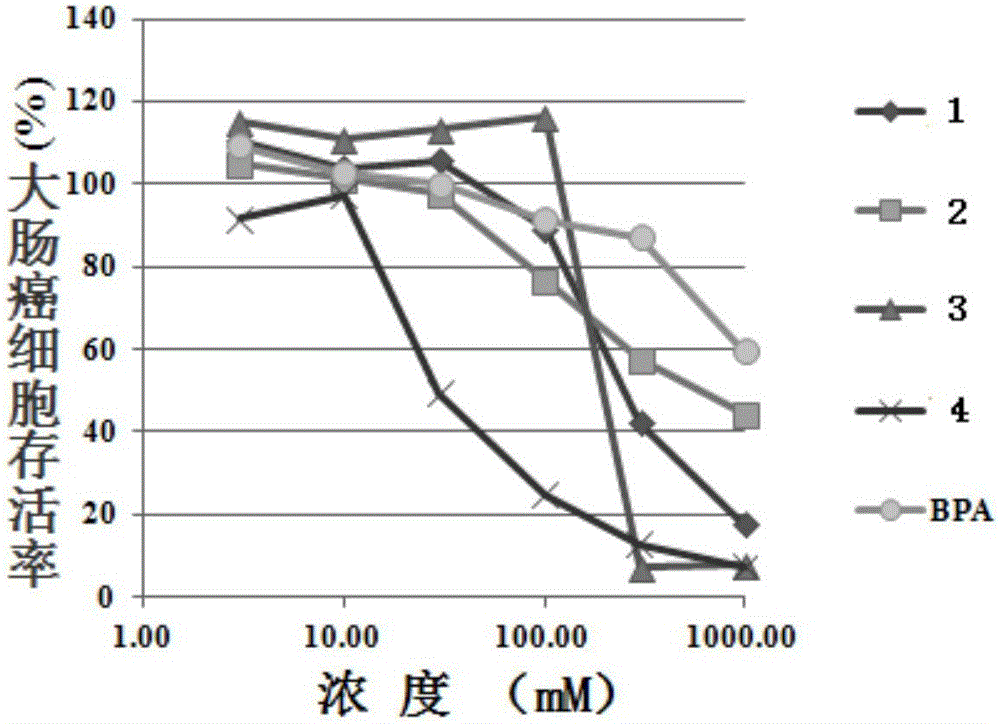
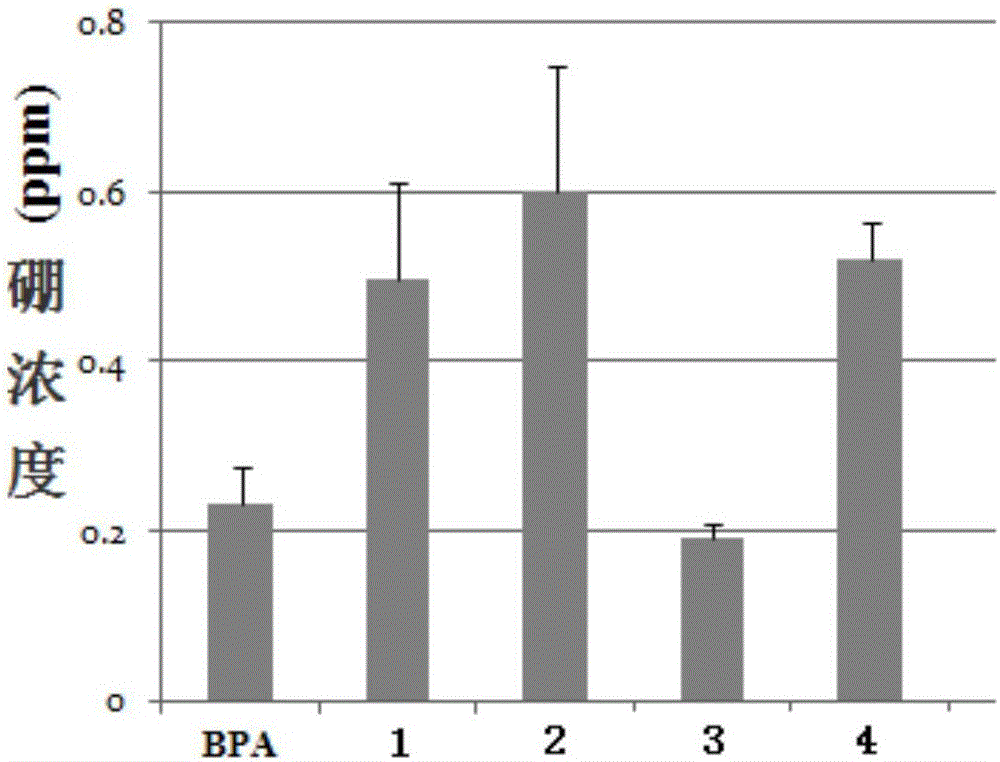
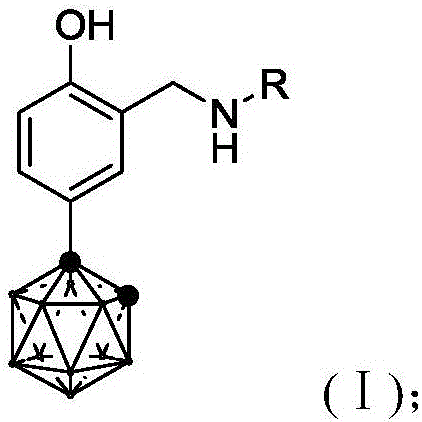
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 compound 1 (2-methylbisglutamic acid phenol borane methyl ester)
[0037] Method 1: Weigh 0.5 g of 2-formylphenol borane (Tetrahedron Letters, 50 (2009) 2960–2963), add L-glutamic acid dimethyl ester hydrochloride (CAS No. 23150-65-4) 0.5 g, added to a two-necked flask and dissolved in 100ml of p-xylene, added 0.18g of sodium cyanoborohydride, 3 drops of formic acid, and the temperature was raised to 150°C for 48 hours. After the reaction was completed, the temperature dropped to room temperature. Distill the solvent under reduced pressure, add 100ml of distilled water and 100ml of dichloromethane for extraction, and add MgSO to the obtained organic phase 4 Dry, filter the mother liquor and distill under reduced pressure to obtain 0.48g, 2-methyl bisglutamic acid phenol borane methyl ester = yield 46%, calculated as 2-formyl phenol borane. The detection parameters are:
[0038] 1 H-NMR: 2.77(t, J=5.5Hz, 2H), 3.26(s, 3H), 3.43(t, J=5.5H...
Embodiment 2
[0041] The preparation of embodiment 2 compound 2 (preparation of 2-methylglutamic acid phenol borane methyl ester)
[0042] Method 1: Weigh 0.5g of 2-formylphenol borane, add 0.25g of L-dimethylglutamate hydrochloride, add it into a two-necked bottle and dissolve it in 100ml of p-xylbenzene, add cyanide 0.18g of sodium borohydride, 3 drops of formic acid, and the temperature was raised to 150°C for 48 hours. After the reaction was completed, the temperature was lowered to room temperature, the solvent was distilled under reduced pressure, and 100ml of distilled water and 100ml of dichloromethane were added for extraction. The obtained organic phase was added to MgSO 4 Dry, filter the mother liquor and distill under reduced pressure to obtain 0.47g, 2-methyl glutamic acid phenol borane methyl ester = yield 59%, calculated as 2-formyl phenol borane.
[0043] 1 H-NMR: 2.68(t, J=5.5Hz, 2H), 3.28(s, 3H), 3.41(t, J=5.4Hz, 2H), 3.45(s, 3H), 3.88(s, 2H), 5.06 (s, 1H), 6.68–6.69 (d...
Embodiment 3
[0046] The preparation of embodiment 3 compound 3 (2-methylaspartic acid phenol borane methyl ester)
[0047] Method 1: Weigh 0.5g of 2-formylphenol borane, add 0.46g of L-aspartic acid dimethyl ester hydrochloride (CAS No. 32213-95-9), add it into a two-necked bottle and dissolve in Add 0.18 g of sodium cyanoborohydride and 3 drops of formic acid to 100 ml of p-xylene, and raise the temperature to 150°C for 48 hours. Chloromethane 100ml is extracted, and the organic solvent obtained adds MgSO 4 Dry, filter the mother liquor and distill under reduced pressure to obtain 0.55 g, 2-methylaspartic acid phenol borane methyl ester = yield 71%, calculated as 2-formyl phenol borane. The detection parameters are:
[0048] 1 H-NMR: 2.71(s,2H), 3.24(s,3H), 3.40(s,2H), 3.78(s,3H), 5.01(s,1H), 6.65–6.67(d, J=8.5Hz, 1H), 7.29 (s, 1H), 7.33–7.35 (d, J=8.6Hz, 1H).
[0049] Method 2: Weigh 0.5g of 2-formylphenol borane, add 0.46g of L-aspartate dimethyl hydrochloride, add it into a two-ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com