A method for detecting related substances in tauroursodeoxycholic acid
A technology of tauroursodeoxycholic acid and related substances, which is applied in the field of high-performance liquid chromatography-evaporative light scattering detection method, can solve the problems of low sensitivity, low accuracy, and interference of components to be measured, and achieve a reduction in chromatographic The effect of peak broadening behavior, good specificity and accuracy, and improved quality controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 Determination of chromatographic column
[0047] Preliminary mobile phase: mobile phase A-mobile phase B;
[0048] Mobile phase A is 50mmol / L ammonia water (adjust the pH to 3.5 with formic acid), and mobile phase B is acetonitrile;
[0049] The column temperature is 40°C;
[0050] The flow rate is 1.0ml / min;
[0051] Evaporative light scattering detector, drift tube temperature is 115°C, carrier gas flow rate is 2.5L / min;
[0052] The test solution: take an appropriate amount of tauroursodeoxycholic acid raw material, and prepare a solution of about 10 mg / ml to obtain the product;
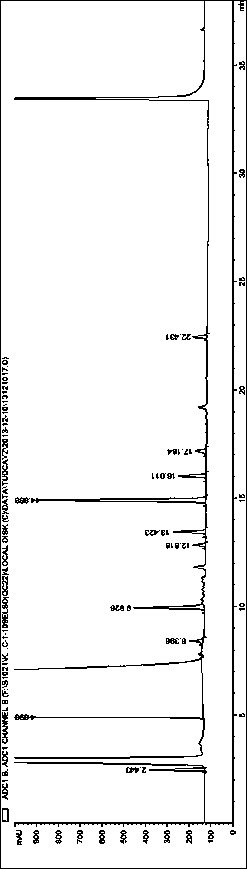
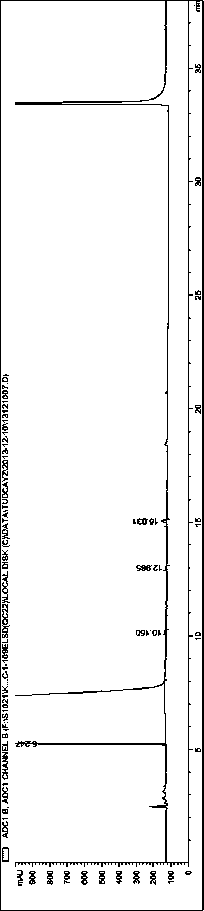
[0053]System Suitability Solution: Take Tauroursodeoxycholic Acid, Tauro-7-ketocholic Acid, Taurochenodeoxycholic Acid, Taurocholic Acid, Cholic Acid, Ursodeoxycholic Acid, 7-Ketocholic Acid Appropriate amounts of cholelithic acid, chenodeoxycholic acid, and cholelithic acid reference substances were prepared into a mixed solution containing about 250 μg / ml of each componen...
Embodiment 2
[0058] The selection of embodiment 2 mobile phase
[0059] Accurately measure tauroursodeoxycholic acid reference solution 1 (2.5 μg / ml) or tauroursodeoxycholic acid reference solution 2 (5.0 μg / ml) and system suitability solution respectively, under different mobile phase conditions Next, inject into the high performance liquid chromatograph and record the chromatogram. Investigate the effects of different types and concentrations of buffer salts and different pH regulators on component separation (the number of system suitability solution peaks) and detection sensitivity. The results are shown in Table 2:
[0060] Table 2 Buffer salt selection results
[0061]
[0062] Column: The chromatographic column number is the same as in Table 1.
[0063] Above-mentioned result shows: adopt 2.5mmol / L~50mmol / L ammonium salt buffer solution, adjust pH value to in the scope of 2.5~3.5 with formic acid, acetic acid or trifluoroacetic acid, can obtain higher sensitivity, can guarantee...
Embodiment 3
[0077] Example 3 Determination of Evaporative Light Scattering Detector Conditions
[0078] Chromatographic conditions:
[0079] Chromatographic column: with the chromatographic column 3 in embodiment 1;
[0080] Mobile phase: with buffer salt 6 in embodiment 2;
[0081] Gradient elution conditions:
[0082]
[0083] Flow rate: 1.0ml / min;
[0084] The injection volume is 10 μl;
[0085] Investigate the influence of evaporative light scattering detector conditions, drift tube temperature in the range of 70-115 °C, and carrier gas flow rate in the range of 2.5-4.0 L / min, take 10 μg / ml tauroursodeoxycholic acid reference solution 10 μl respectively Inject into a liquid chromatograph, record the chromatogram, and investigate the sensitivity of tauroursodeoxycholic acid response under different conditions. Other chromatographic conditions are as above, and the results are shown in Table 7.
[0086] Table 7 Effect of evaporative light scattering detector conditions on sensit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com