Regeneration method of paraffin dehydrogenation catalyst
A dehydrogenation catalyst and alkane dehydrogenation technology, applied in the direction of catalyst regeneration/reactivation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of affecting the performance of low-carbon alkane dehydrogenation regeneration catalysts It has the effect of improving regeneration stability, easy operation, and prolonging the service life and service life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
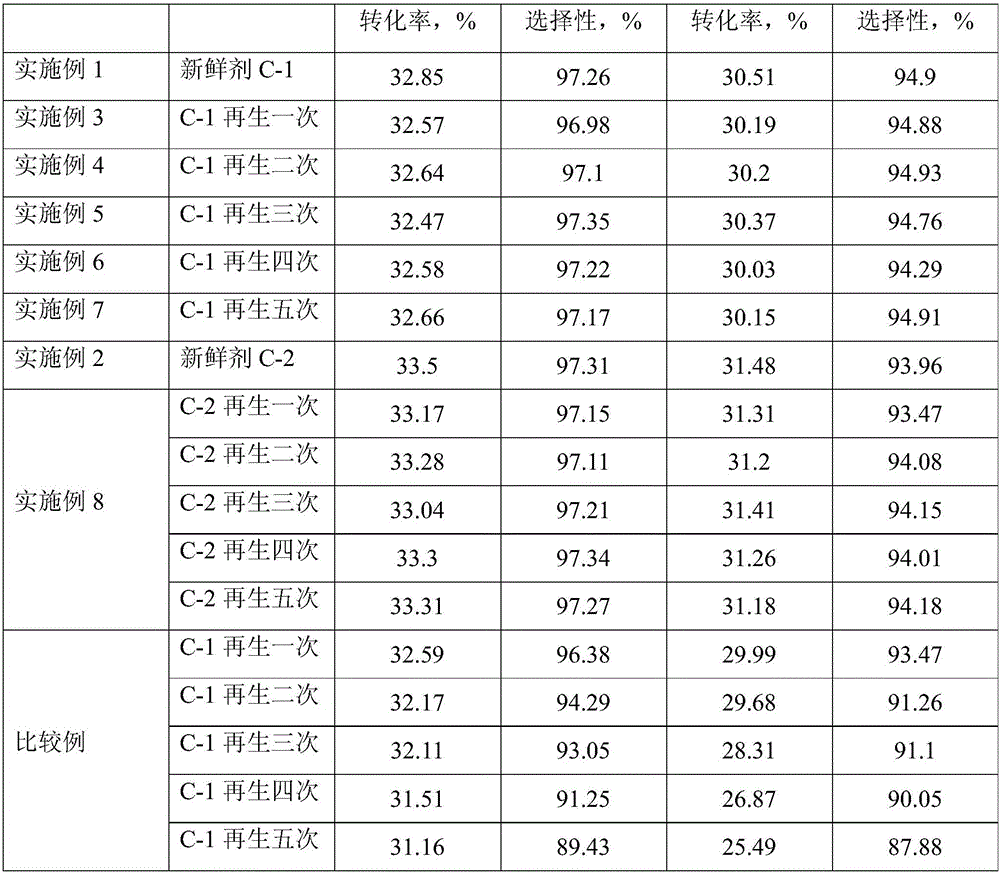
[0030] Weigh a commercially available alumina carrier (γ phase, spherical, 0.5 mm in diameter, 0.71 cm in pore volume) 3 / g, specific surface area 224m 2 / g) 30g, deionized water was added dropwise to the initial moistening, and the volume of water consumed was 27mL. According to the weight content of Sn element in the final catalyst of 0.4%, stannous chloride containing 0.12g Sn was weighed and dissolved in ethanol, and the volume was made up to 27mL with ethanol. The prepared Sn-containing ethanol solution was added to 30 g of alumina carrier, mixed evenly, and aged at room temperature for 2 hours. It was dried at 80°C for 8h, and then calcined at 600°C for 4h.
[0031] According to the weight content of Pt element in the final catalyst of 0.5%, chloroplatinic acid containing 0.18 g of Pt was weighed and dissolved in deionized water, the volume was adjusted to 27 mL, added to the alumina carrier containing Sn, mixed evenly, and aged at room temperature for 4 h. Dry at 100...
Embodiment 2
[0034] Weigh a commercially available alumina carrier (γ phase, spherical, 0.5 mm in diameter, 0.71 cm in pore volume) 3 / g, specific surface area 224m 2 / g) 30g, deionized water was added dropwise to the initial moistening, and the volume of water consumed was 27mL. According to the weight content of Sn element in the final catalyst of 0.6%, stannous chloride containing 0.18g Sn was weighed and dissolved in ethanol, and the volume was made up to 27mL. The prepared Sn-containing ethanol solution was added to 30 g of alumina carrier, mixed evenly, and aged at room temperature for 4 hours. It was dried at 100°C for 6h, and then calcined at 500°C for 6h.
[0035] According to the weight content of Pt element in the final catalyst of 0.7%, chloroplatinic acid containing 0.12 g of Pt was weighed and dissolved in deionized water, and the volume was adjusted to 27 mL. Dry at 120°C for 4h and calcinate at 500°C for 6h. The samples obtained in the above steps were treated at 700°C ...
Embodiment 3
[0038] After the C-1 fresh agent reacted for 72 hours, the feed gas was switched to pure hydrogen gas and purged for 1 hour; then the temperature was lowered to 470°C, switched to a nitrogen-oxygen mixed gas with an oxygen content of 15v%, and treated for 2 hours; then the temperature was raised to 530°C , switch to a nitrogen-oxygen mixed gas with an oxygen content of 21v%, and treat for 2h; then continue to heat up to 580 °C, switch to a nitrogen-oxygen mixed gas with an oxygen content of 35v%, and treat for 3h.
[0039] After the charcoal is burned, switch to nitrogen, cool down to 400 ° C, and introduce a nitrogen-oxygen mixed gas with an oxygen content of 10v%, and H 2 The molar ratio of O / Cl is 60:1 containing carbon dichloride water vapor, the carbon dichloride water vapor is introduced for 10h, and the total amount of water vapor introduced is 4000 μg per gram of catalyst.
[0040] After the oxychlorination of the deactivated catalyst was updated, it was switched to ni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com