A method for preparing ferrous oxalate of specific crystals by using waste liquid of cold-rolled steel hydrochloric acid pickling
A technology for ferrous oxalate and pickling waste liquor, which is applied in the fields of carboxylate preparation, carboxylate preparation, organic chemical methods, etc. Problems such as unstable quality of iron and lithium, to achieve the effect of low cost, small particle size and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
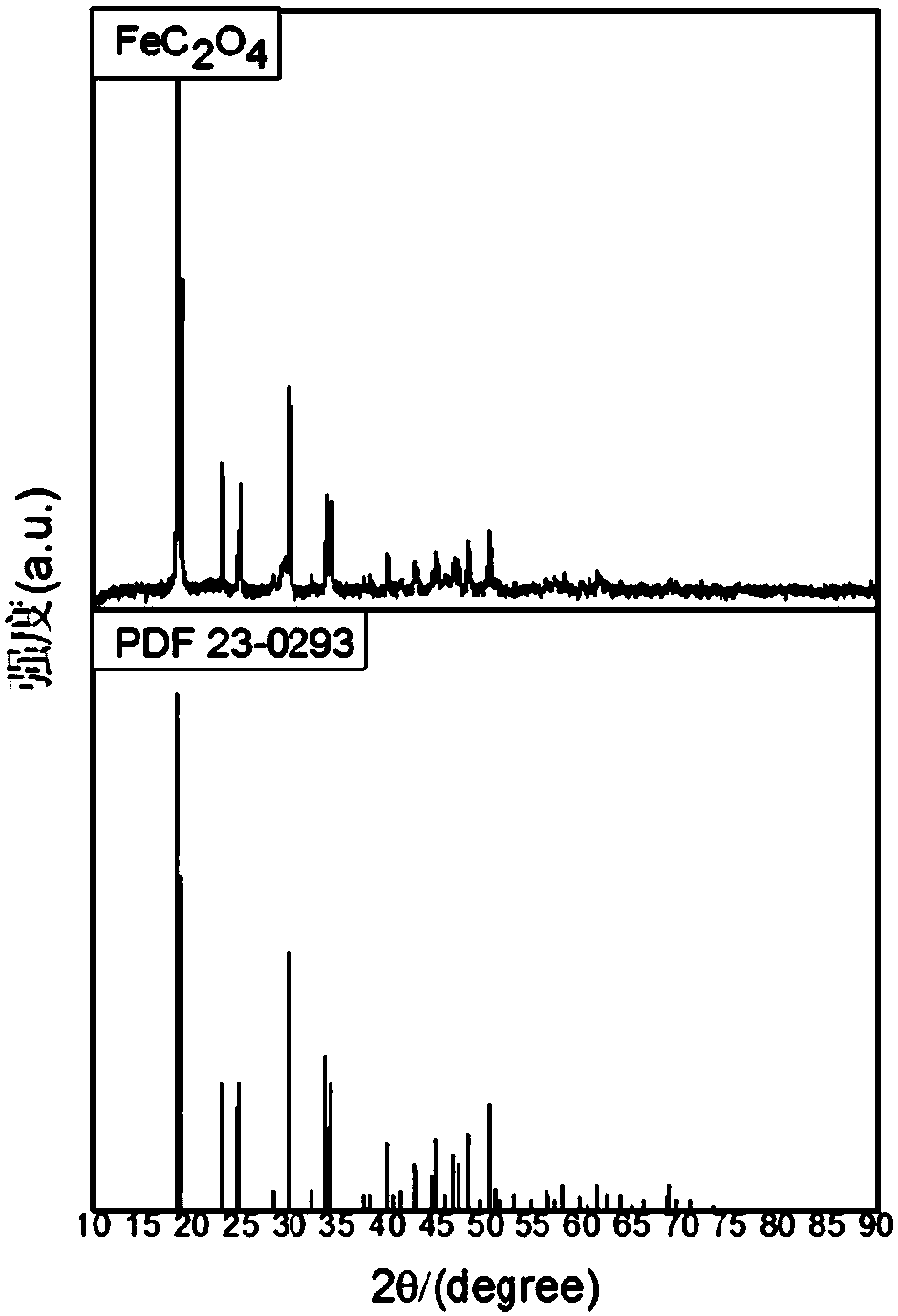
[0032] Prepare α-type ferrous oxalate, the steps are as follows:
[0033] (1) Pretreatment of waste hydrochloric acid pickling of cold-rolled steel: heating up the waste hydrochloric acid pickling of cold-rolled steel to more than 85°C, adding excess iron filings, and adjusting the ferrous ion content in the solution to 180g / L, Adjust the pH value to 2.5, use a straw to remove the black oily substance produced on the surface of the solution, stir and react at a constant temperature of 85°C for 6 hours, add hydrochloric acid and water to make the content of hydrochloric acid in the solution 100g / L, and filter to remove impurities after standing for 24 hours;
[0034] (2) Generate ferrous hydroxide suspension: Add ammonia or calcium oxide to the solution treated in step (1), adjust the pH value of the solution to 10.7, control the reaction temperature to 85°C, and generate ferrous hydroxide suspension ;
[0035] (3) Generate ferrous oxalate precipitation: control the reaction t...
Embodiment 2
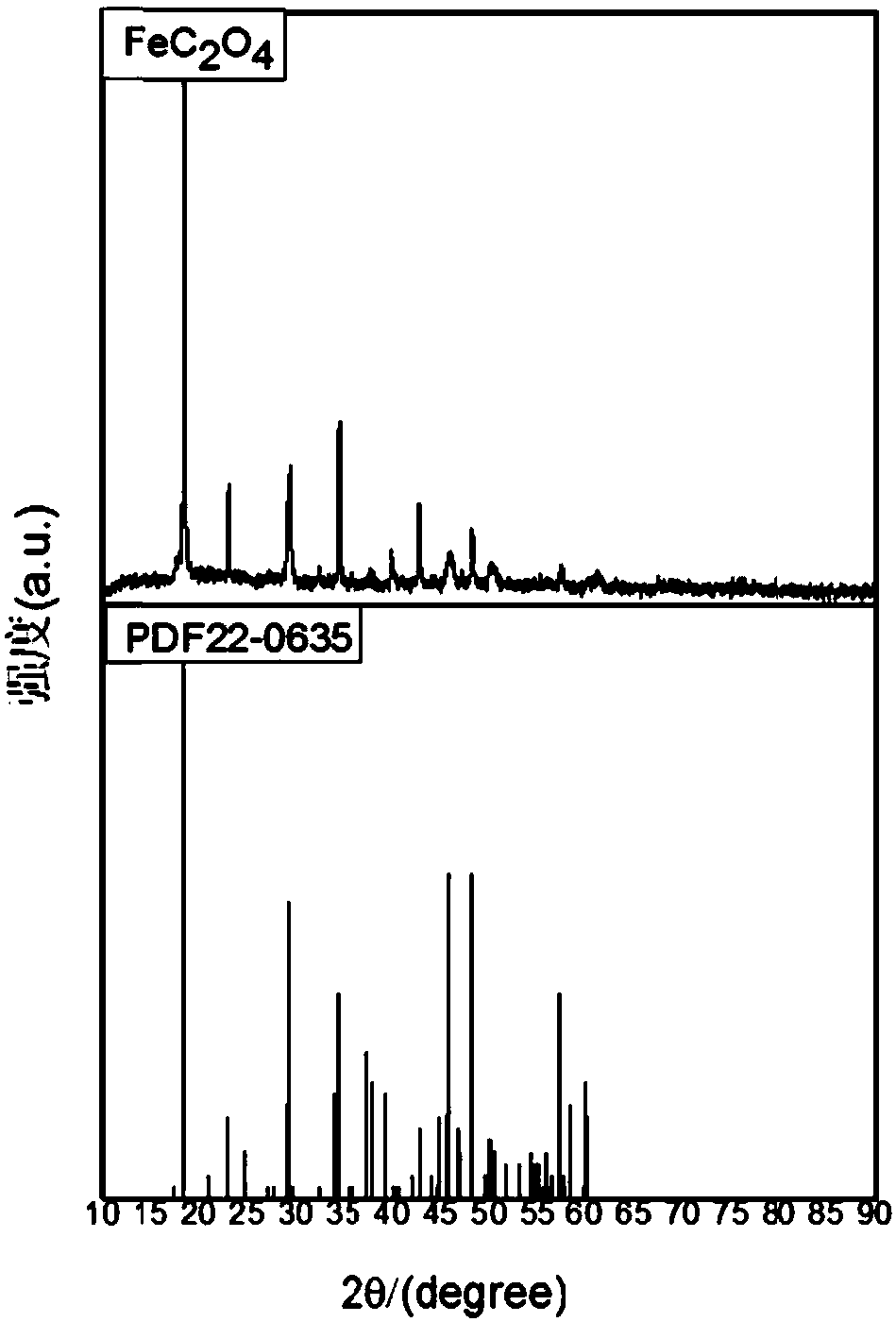
[0039] Preparation of β-type ferrous oxalate, the steps are as follows:
[0040] (1) Pretreatment of waste hydrochloric acid pickling of cold-rolled steel: heating up the waste hydrochloric acid pickling of cold-rolled steel to more than 85°C, adding excess iron filings, and adjusting the ferrous ion content in the solution to 180g / L, Adjust the pH value to 2.5, use a straw to remove the black oily substance produced on the surface of the solution, stir and react at a constant temperature of 85°C for 6 hours, add hydrochloric acid and water to make the content of hydrochloric acid in the solution 50-100g / L, and filter it after standing for 24 hours miscellaneous;
[0041] (2) Generate ferrous hydroxide suspension: Add ammonia or calcium oxide to the solution treated in step (1), adjust the pH value of the solution to 5.8, control the reaction temperature to 30°C, and generate ferrous hydroxide suspension ;
[0042] (3) generate ferrous oxalate precipitation: the control reacti...
Embodiment 3
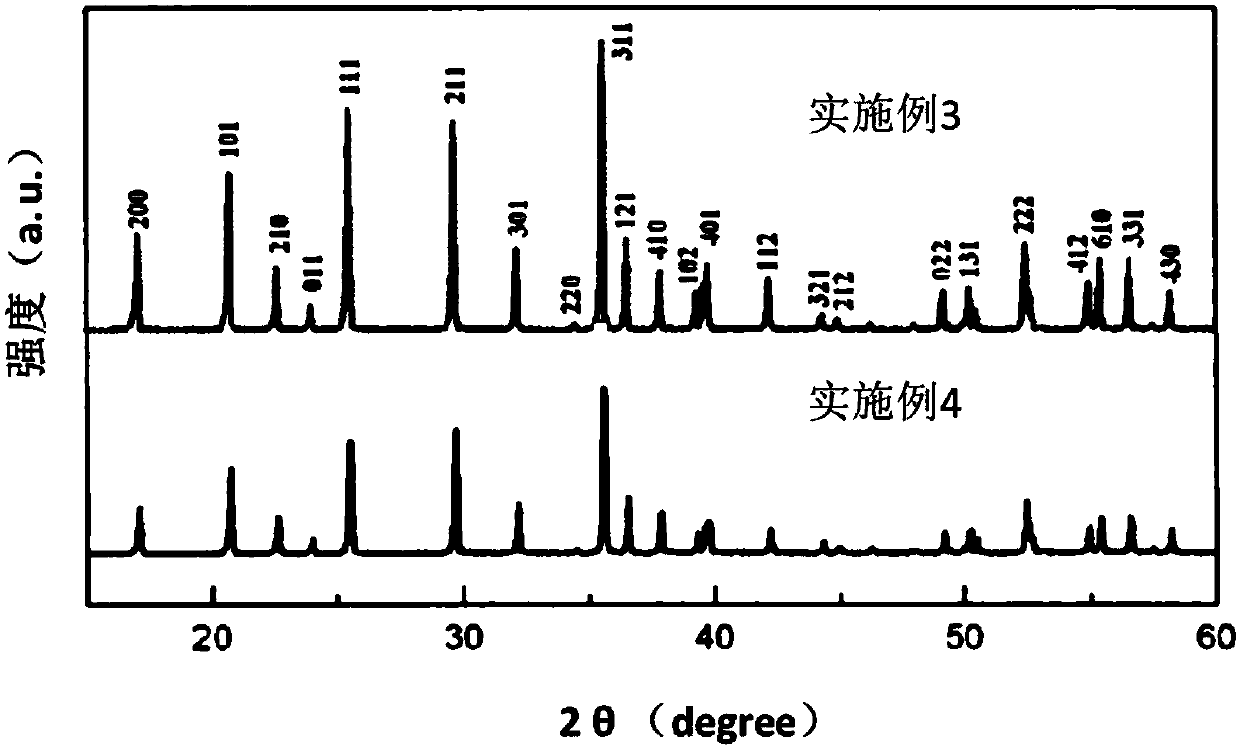
[0047] Example 3: Preparation of lithium iron phosphate by using α-type ferrous oxalate
[0048] Weigh 0.01M lithium hydroxide, 0.01M α-type ferrous oxalate (prepared according to Example 1), add glucose with a weight ratio of 5% of the above reaction substances, add 25ml of ethanol to dissolve, stir for 24h, and dry at 60°C. Finally, the above mixture was calcined at 700° C. for 10 h in an argon atmosphere, and cooled naturally to room temperature to obtain lithium ferrous phosphate.
[0049] Through material characterization, it was found that the (111) diffraction peak of lithium iron phosphate prepared from α-type ferrous oxalate was higher than the (211) diffraction peak, indicating that there was no dislocation and mixing of Li and Fe atoms. In addition, the higher diffraction intensity of the sample shows that the lithium ferrous phosphate prepared by α-type ferrous oxalate has better crystallinity (see image 3 ).
[0050] Through the electrochemical performance test...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com