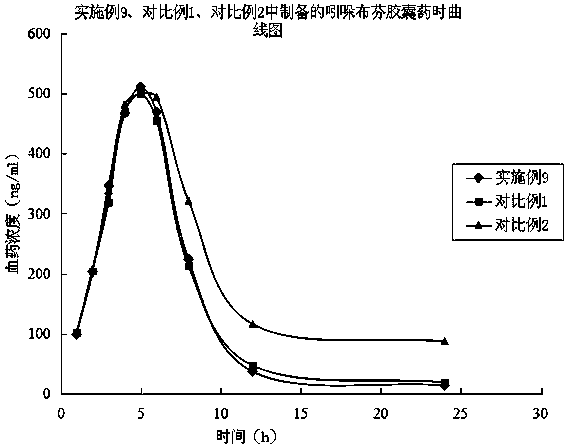
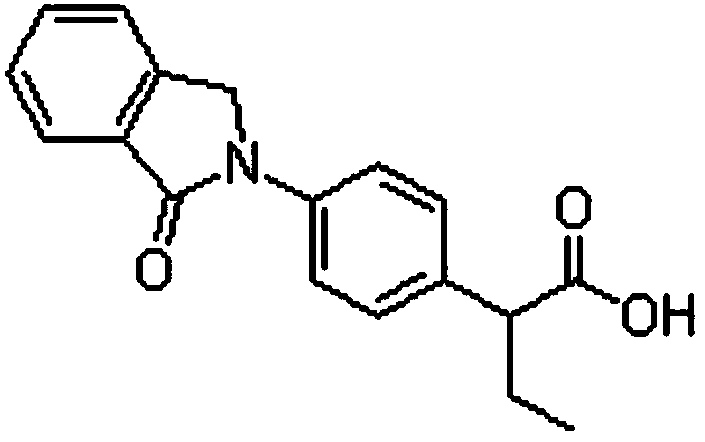
A kind of indobufen solid preparation and preparation method thereof
A technology of indobufen and solid preparations, which is applied in the fields of pill delivery, pharmaceutical formulations, and medical preparations of non-active ingredients, etc., which can solve the problems of high technical requirements for production equipment and personnel, and increase the risk of frequent gastrointestinal disturbances , unstable clinical bioavailability and other issues, to achieve clear safety performance, reduce bleeding events and gastrointestinal disorders, and change solubility and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
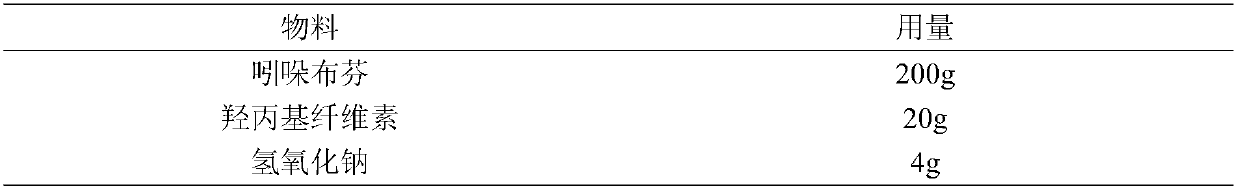
[0033] Embodiment 1 Preparation of indobufen complex
[0034] Indobufen Compound Prescription List
[0035]
[0036] Preparation Process:
[0037] Dissolve 4g of pharmaceutical grade sodium hydroxide in 100ml of purified water and stir to dissolve to prepare sodium hydroxide solution for later use;
[0038] 1. Dissolve 20g of hydroxypropyl cellulose in 50g of ethanol solution and stir until it is completely dissolved, then slowly add 200g of indobufen, stir until it dissolves evenly, then slowly add sodium hydroxide solution dropwise, stirring while adding Evenly, while monitoring the pH value change of the solution, when the pH value of the solution is 8.0, stop the dropwise addition to obtain a complex solution of indobufen;
[0039] 2. Place the indobufen complex solution in step 1 in the colloid mill equipment, control the flow rate to 0.3L / h, output 1000rpm, and line speed 15m / s, let the compound solution accumulate and grind in the colloid mill for 50min, Grinding ...
Embodiment 2
[0041] Example 2 Preparation of Indobufen Complex
[0042] Indobufen Compound Prescription List
[0043]
[0044] Preparation Process:
[0045] Dissolve 4g of pharmaceutical grade sodium hydroxide in 100ml of purified water and stir to dissolve to prepare a sodium hydroxide solution for later use;
[0046]1. Dissolve 20g of hydroxypropyl cellulose in 40g of purified aqueous solution and stir until completely dissolved, then slowly add 100g of indobufen, stir until it dissolves evenly, then slowly add sodium hydroxide solution dropwise, stirring while adding Evenly, while monitoring the pH value change of the solution, when the pH value of the solution is 7.5, stop the dropwise addition to obtain a complex solution of indobufen;
[0047] 2. Put the indobufen complex solution in step 1 in the colloid mill, control the flow rate to 0.6L / h, output 1500rpm, and line speed 25m / s, and let the compound solution accumulate and grind in the colloid mill for 40min. Grinding ends an...
Embodiment 3
[0049] Example 3 Preparation of Indobufen Complex
[0050] Indobufen Compound Prescription List
[0051]
[0052] Preparation Process:
[0053] Dissolve 8.5g of pharmaceutical-grade sodium carbonate in 100ml of purified water and stir to dissolve to prepare a sodium carbonate solution for subsequent use;
[0054] 1. Dissolve 300g of hydroxypropyl cellulose in 300g of 60% ethanol aqueous solution and stir until completely dissolved, then slowly add 300g of indobufen, stir until it dissolves evenly, then slowly add sodium carbonate solution dropwise, while adding While stirring evenly, monitor the pH value change of the solution at the same time, and when the pH value of the solution is 7.9, stop the dropwise addition to obtain a complex solution of indobufen;
[0055] 2. Place the indobufen complex solution in step 1 in the colloid mill, control the flow rate to 0.8L / h, output 1800rpm, and line speed 22m / s, and let the compound solution accumulate and grind in the colloid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com