Method for preparing sulfonic acid group-modified mesoporous material-loaded heteropolyacid catalyst and application thereof during esterification reaction
A technology for loading heteropolyacids and mesoporous materials, which is applied in catalytic reactions, preparation of organic compounds, preparation of carboxylate esters, etc., can solve the problems of easy dissolution and desorption of active components, and achieves simple preparation process, strong acidity, and large pores. the effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
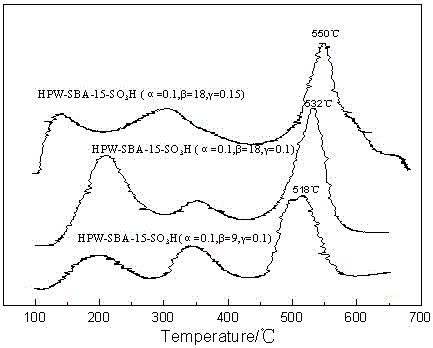
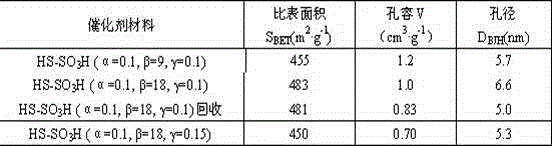
[0026] Dissolve 4g of P123 in 120g of 2mol / L hydrochloric acid solution, stir at 35°C for 3 hours to completely dissolve the templating agent; raise the temperature to 40°C, and add 8.42g of tetraethyl orthosilicate (TEOS) dropwise to the above solution, Continue to stir for 60 minutes; add 3-mercaptopropyltrimethoxysilane (MPTMS), phosphotungstic acid (HPW) and hydrogen peroxide solution (30wt%) to the above solution in sequence, molar composition α=n(MPTMS) / n(MPTMS +TEOS)=0.1, β=n(H 2 o 2) / n(MPTMS)=9, mass component γ=m(HPW / m(TEOS)=0.1, under nitrogen protection, continue to stir for 20h, then crystallize at 100°C for 24h, filter, wash with ethanol and aqueous solution respectively , vacuum-dried at 80°C for 12h, ethanol Soxhlet extraction for 24h, vacuum-dried at 80°C for 12h, and the obtained sample was designated as HPW-SBA-15-SO 3 H (α=0.1, β=9, γ=0.1).
Embodiment 2
[0028] The application of a heteropolyacid catalyst supported by a sulfonic acid group-modified mesoporous material in esterification reaction. Weigh 1.23g of heteropoly acid catalyst HPW-SBA-15-SO 3 H (α=0.1, β=9, γ=0.1), then weighed 8.21g cyclohexene and 13.81g formic acid into a 50ml three-necked flask, stirred at 80°C for 6h, cooled to room temperature, and used gas chromatography Analysis of the components of the reaction solution showed that the conversion rate of cyclohexene was 82%, and the selectivity of cyclohexyl formate was 99%.
Embodiment 3
[0030] Loading heteropolyacid catalyst HPW-SBA-15-SO on recycled sulfonic acid group-modified mesoporous materials 3 H (α=0.1, β=9, γ=0.1) was vacuum-dried at 100°C for 12 hours, cooled to room temperature, weighed 1.23g, then weighed 8.21g cyclohexene and 13.81g formic acid and added them to a 50ml three-necked flask in turn, Stirring at 80° C. for 6 h, cooling to room temperature, and analyzing the components of the reaction solution by gas chromatography showed that the conversion rate of cyclohexene was 83%, and the selectivity of cyclohexyl formate was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com