Method for preparing feed calcium hydrophosphate from calcium source in phosphorite
A technology of calcium hydrogen phosphate and calcium dihydrogen phosphate, applied in animal feed, chemical instruments and methods, applications, etc., can solve the problems of phosphogypsum treatment and stockpiling, environmental protection and economic development needs obstacles, and achieve significant economic and social benefits , good economic and social benefits, and the effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] High-temperature phosphate rock decomposes fluoride to produce calcium phosphate:
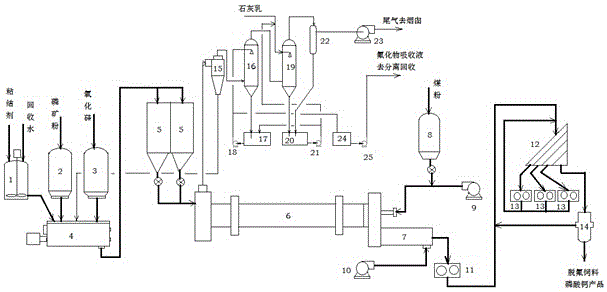
[0056] Such as figure 1 Shown: first binder and recovery process water are made into the concentration of 2-5% in binder preparation tank 1; Cross 100 order fineness phosphate rock powder (composition is shown in Table 2) from phosphate rock powder storage hopper 2 and The silicon oxide from the silicon oxide storage hopper 3 (the silicon oxide content in the phosphate rock is higher than 5%, can not be added) is continuously added to the twin-shaft kneading granulator 4 through the speed-regulating metering belt conveyor, where it is mixed with the binder by the binder. The binder configured in the groove and metered by the flow meter is sprayed onto the material in the twin-shaft kneading granulator 4, kneaded, extruded and rolled into granules. The granules granulated from the granulator have a particle size of Φ4-6mm and a moisture content of 10-12%, and are sent into the feeding bi...
Embodiment 2
[0069] Wet process phosphoric acid precipitation defluorination production:
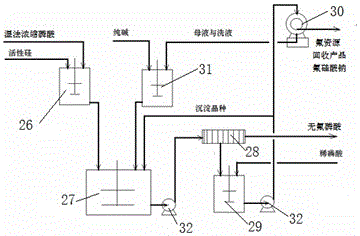
[0070] Such as figure 2As shown, 50,000 kg / h of concentrated phosphoric acid (see Table 4 for composition) is pumped into the silicon dissolution tank 26, and after adding 140 kg / h of active silicon oxide for dissolution, it is sent into the fluorine precipitation tank 27, and fluorine-containing sodium silicate is added 1400kg / h of 20% seed crystal slurry and 3130kg / h of the mixed solution of 17% sodium dihydrogen phosphate and phosphoric acid sent by the alkali dissolution tank were carried out at normal temperature for 60 minutes of stirring precipitation reaction.
[0071] Reaction generates material 54670kg / h in the fluorine precipitation tank 27, is sent in the filter press 28 by filter press pump and carries out solid-liquid separation, obtains filtrate 53420kg / h (44.70% P 2 o 5 , 0.09% F) and 1250kg / h of solid-containing 80% filter cake; the filtrate is the semi-finished product defluoroph...
Embodiment 3
[0079] Feed calcium hydrogen phosphate production:
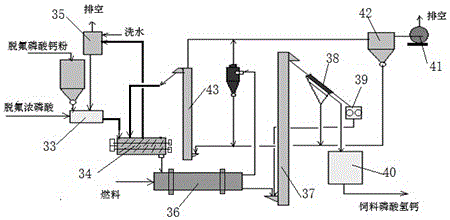
[0080] Such as image 3 As shown, defluorinated concentrated phosphoric acid (composition table 5) 3770kg / h and finely prepared tricalcium phosphate powder (composition is shown in table 3) 5000kg / h, add high-speed mixer 33 and mix rapidly together, add 800kg / h After h process water, enter the biaxial kneading and granulation reactor 34, and add fine product return materials at the same time, carry out metathesis reaction on the one hand, and knead and granulate by using the biaxial rotary vane on the other hand; the dust-containing gas released by the reaction is The dust that adds water washing recovery wherein of device 35, washing water consumption and adding 800kg / h amount carry out balance regulation.
[0081] After the kneading and granulation reaction is completed, the wet product containing 5-8% enters the rotary drum dryer 36, and is dried in parallel contact with the hot air from the gas combustion furnace. The d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com