Preparation method and application of organic conjugated micro-molecules rich in nitrogen and sulfur
An organic field and reaction technology, applied in the fields of organic chemistry, semiconductor/solid-state device manufacturing, electrical components, etc., can solve the problems of lagging development of n-type materials, and achieve the effect of good application prospects, good electron mobility, and simple synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of 5,9-dihydro-2,3,7,8-tetrachloropyrazino[2,3-g]quinoxaline compound shown in embodiment 1, formula III
[0038] 1) Synthesis of 5,9-dihydro-1,4,6,9-tetrahydro-pyrazino[2,3-g]quinoxaline-2,3,7,8-tetraketone
[0039] Add 5.1 g of 1,2,4,5-benzenetetraaminotetrahydrochloride into 10% dilute hydrochloric acid solution with 3.8 g of oxalic acid dissolved in it at room temperature, and stir the nitrogen mixture for 0.5 hour; then heat to 100°C and stir After reacting for 12 hours, cool to room temperature, a brown solid precipitated out, suction filtered, washed with distilled water several times until the pH value of the extracted water was about 7.0, then vacuum-dried the obtained solid, and weighed to obtain 4.40 g of a brown solid.
[0040] The structural characterization data are as follows:
[0041] Mass Spectrum: [MS(EI)] m / z: 246 (M + ).
[0042] H NMR spectrum: 1 H NMR (400MHz, CDCl 3 ) δ (ppm): 8.0 (s, 4H), 8.09 (s, 2H).
[0043] 2) Synthesis of 5,9-d...
Embodiment 2、5
[0048] Example 2, 5,11-dihydro-2,2'-([1,3]dithiolo[4,5-e]pyrazino[2,3-g][1,3]dithio Alcoho[4,5-b]quinoxaline-2,8-diylidene) dimalononitrile compound (compound DTYM)
[0049] 1) Synthesis of 1,1-dicyanoethylene-2,2-disulfide sodium salt
[0050] Under stirring, slowly add 6.6g of malononitrile into the suspension of 8.0g of sodium hydroxide in 100mL of ethanol, keep the temperature at 10-15°C, after the addition, weigh 7.6g of carbon disulfide dropwise with a syringe Add it to the reaction solution. After the dropwise addition, continue to react under ice bath conditions for 30 minutes, and then gradually rise to room temperature for 2 hours to obtain a yellow viscous solid, which is filtered by suction, washed with absolute ethanol, and then dried to obtain a light yellow solid powder. 17g.
[0051] The structural characterization data are as follows:
[0052] Elemental analysis: Molecular formula: C 4 N 2 S 2 Na; theoretical value: S, 34.4; found value: S, 33.2.
[005...
Embodiment 3
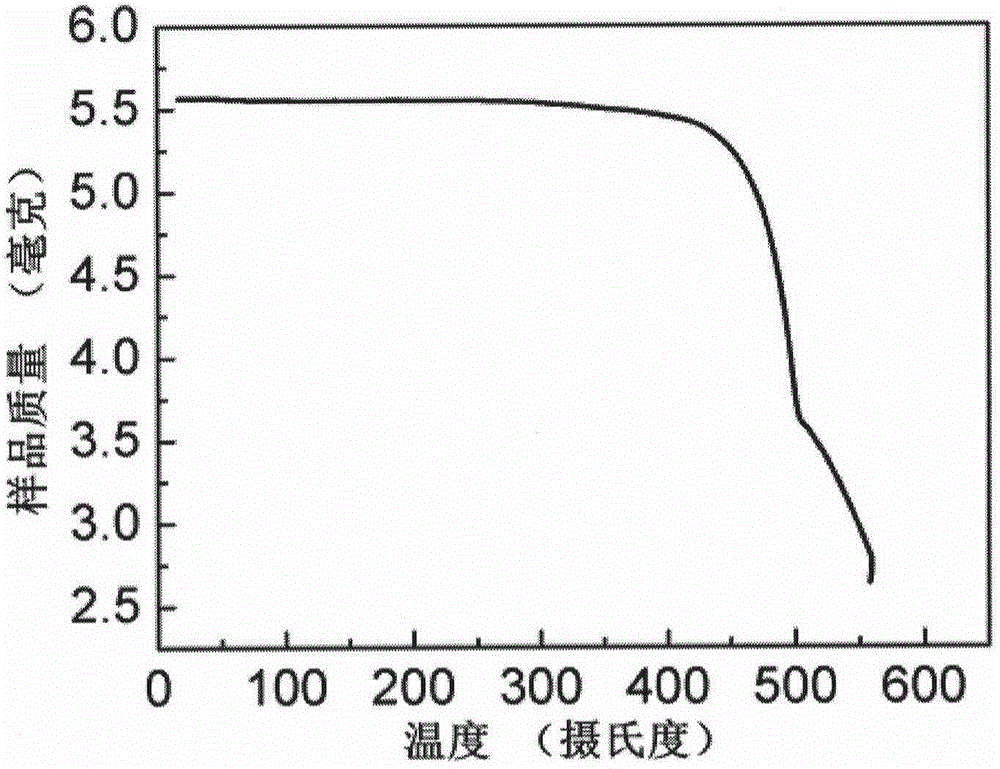
[0058] Embodiment 3, the ultraviolet-visible absorption performance, electrochemical performance, thermal performance and field effect transistor performance of the obtained compound DTYM prepared in embodiment 2
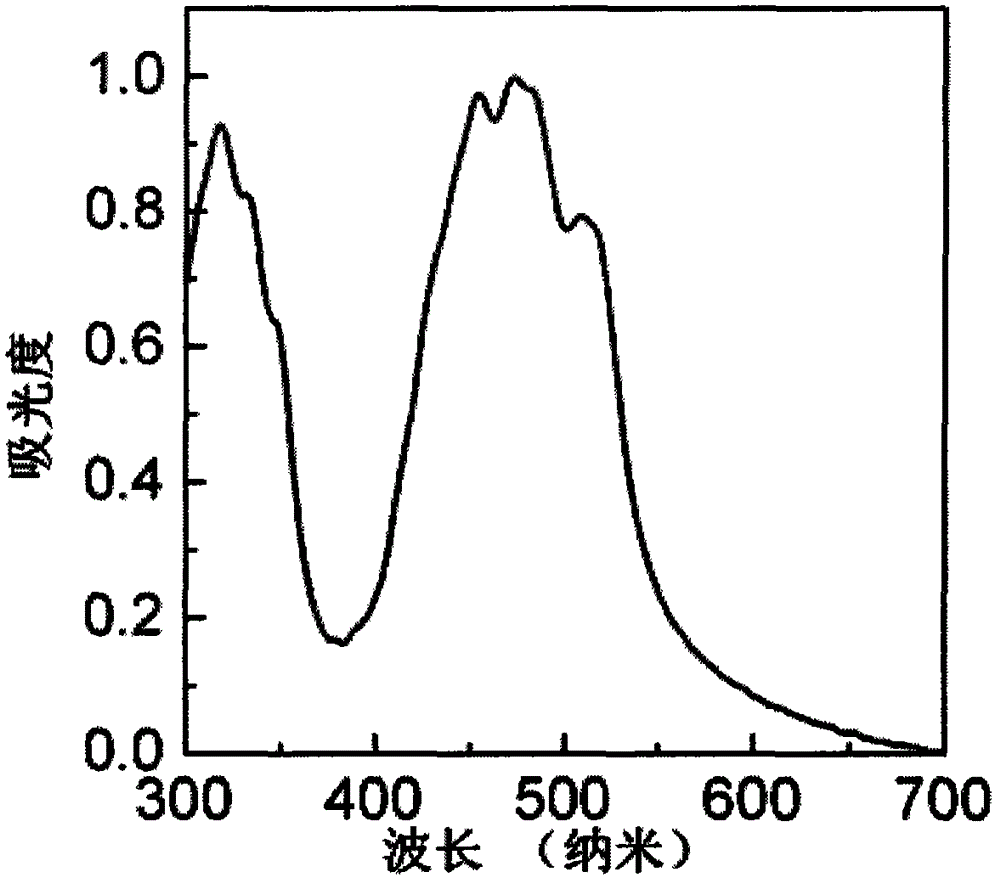
[0059] 1) UV-Vis absorption properties of compound DTYM
[0060] figure 1 is the UV-Vis absorption spectrum of compound DTYM in the film. Depend on figure 1 It can be seen that the maximum absorption peak position of the compound DTYM in the film is 472 nanometers, and the calculated optical band gap is 2.2 electron volts (the optical band gap is calculated according to the formula E g =1240 / λ calculation, where E g is the optical band gap, and λ is the boundary value of the UV absorption curve).
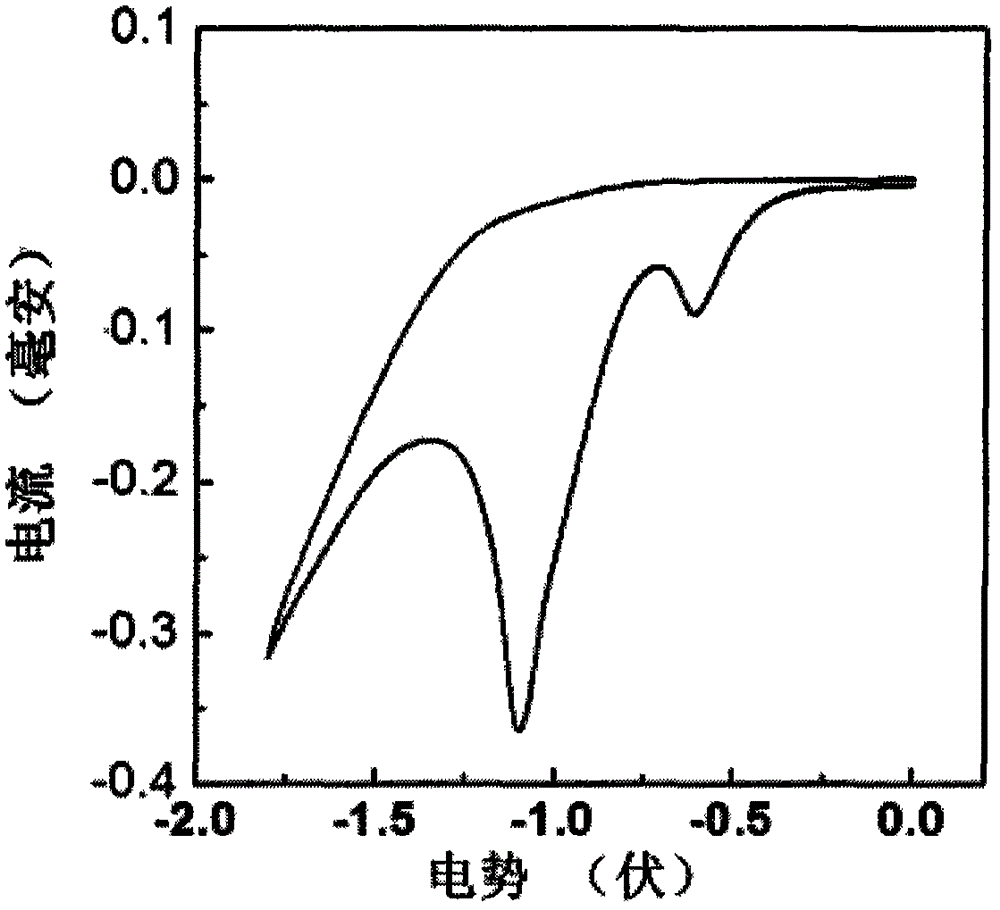
[0061] 2) Electrochemical properties of the compound DTYM
[0062] figure 2 is the cyclic voltammetry curve of compound DTYM. The electrolytic cell adopts a three-electrode system, with platinum as the working electrode, platinum wire as the counter electrode, sil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com