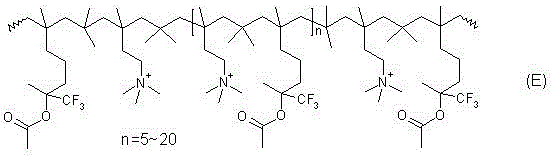
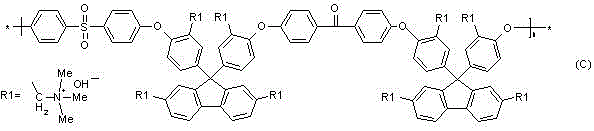
Fuel cell anionic membrane capable of blocking methanol permeation
A fuel cell, anion membrane technology, applied in fuel cells, circuits, electrical components, etc., can solve the problems of inability to verify the service life, difficult to meet service life requirements, etc., and achieve the effect of long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Cationic polymerization: Add 48g of formula A cross-linking monomer (0.2mol) and 42g of formula B monomer (0.4mol) into a 1000ml PTFE-lined autoclave, replace the air with nitrogen for 2~3 times after sealing, and then add isobutylene 112g (2mol). Then, under magnetic stirring, the material was cooled to -30°C, and finally 5ml of boron trifluoride ether was added, the cationic polymerization reaction caused exotherm, and the cooled material was kept at about -20°C. After 2~3 hours, the exotherm ended. Continue to react for 1 hour, the temperature of the material returns to the freezing temperature of the refrigerant, and the polymerization reaction ends to obtain a viscous liquid of polyisobutylene oligomer;
[0045]Amination reaction: Add 200g of absolute ethanol and 45g of dimethylamine (1mol) into the autoclave through a high-pressure dropping device, heat the autoclave with a jacket, keep the autoclave material at 90~100°C, and raise the pressure to 0.3~ 0.5MPa, st...
Embodiment 2
[0051] Cationic polymerization: Add 48g of formula A cross-linking monomer (0.2mol) and 42g of formula B monomer (0.4mol) into a 1000ml PTFE-lined autoclave, replace the air with nitrogen for 2~3 times after sealing, and then add isobutylene 112g (2mol). Then, under magnetic stirring, the material was cooled to -30°C, and finally 5 g of anhydrous hydrogen fluoride was added, and the cationic polymerization reaction caused severe exotherm, which was difficult to control. The cooling material is kept at about -10°C. After 2 to 3 hours, the heat release ends, and the reaction is continued for 1 hour. The temperature of the material returns to the freezing temperature of the refrigerant, and the polymerization reaction ends, and a viscous liquid of polyisobutylene oligomer is obtained;
[0052] All the other operations are identical with embodiment 1;
[0053] After performance testing, the quaternary ammonium salt ion content is 1.69mmol / g, and the membrane conductivity at room ...
Embodiment 3
[0055] Cationic polymerization: Add 48g of formula A cross-linking monomer (0.2mol) and 42g of formula B monomer (0.4mol) into a 1000ml PTFE-lined autoclave, replace the air with nitrogen for 2~3 times after sealing, and then add isobutylene 112g (2mol). Then, under magnetic stirring, the material was cooled to -30° C., and finally 5 g of anhydrous p-toluenesulfonic acid was added, and the exotherm caused by cationic polymerization was very weak. The cooling material is kept at about -20°C. After 4 to 5 hours, the heat release ends, and the reaction is continued for 1 hour. The temperature of the material returns to the freezing temperature of the refrigerant, and the polymerization reaction is completed, and a viscous liquid of polyisobutylene oligomer is obtained;
[0056] All the other operations are identical with embodiment 1;
[0057] After performance testing, the quaternary ammonium salt ion content is 1.12mmol / g, and the membrane conductivity at room temperature is 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com