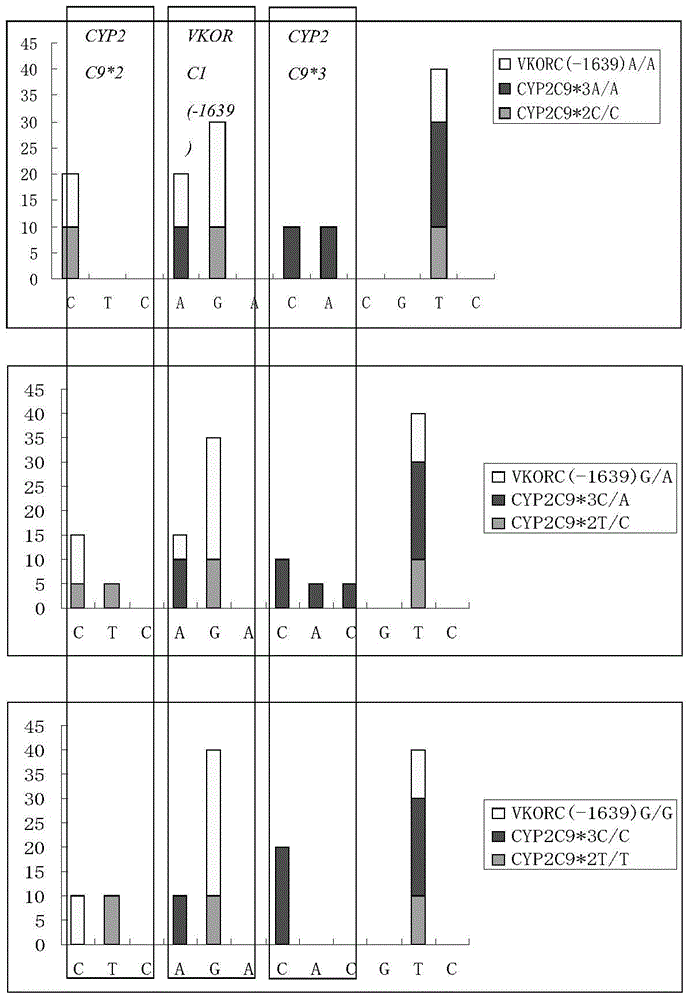
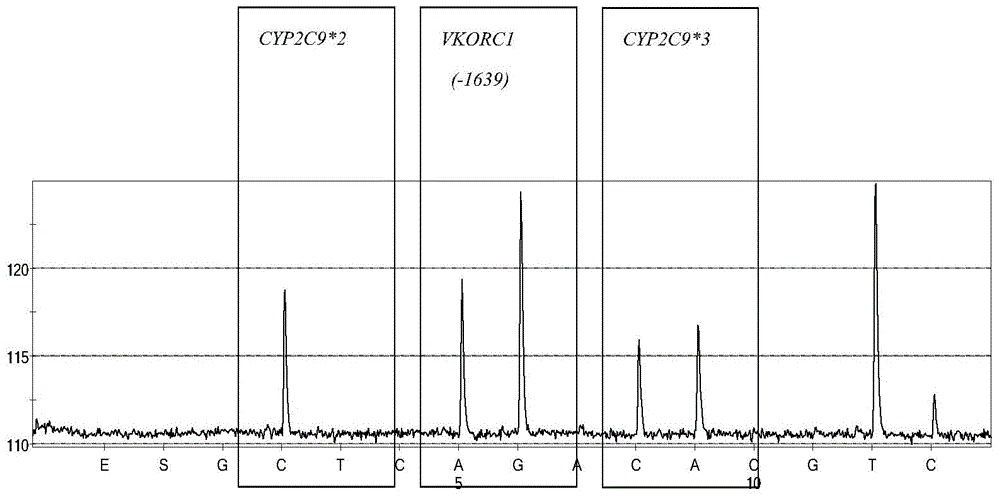
Kit for rapid detection of polymorphism of Warfarin metabolic enzyme gene by virtue of pyrosequencing method and application of kit
A pyrosequencing method, a gene polymorphism technology, is applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc. The effect of small volume and large promotion and application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Reagents.
[0038] (1) DNA extraction reagents:
[0039] Purchased from QIAGEN Company.
[0040] (2) Reaction solution:
[0041] PCR Buffer: purchased from Fermentas, USA;
[0042] Primers SEQ ID NO: 1-6, synthesized by Shanghai Yingjun Biotechnology Co., Ltd.;
[0043] MgCl 2 : purchased from the U.S. Fermentas company;
[0044] 0.2mM dNTPs: purchased from Fermentas, USA;
[0045] 2U / μL Taq DNA polymerase: purchased from Fermentas, USA.
[0046] (3) Reagent for single-strand purification:
[0047] 75% (v / v) ethanol solution: purchased from Hangzhou Changzheng Chemical Reagent Co., Ltd.;
[0048] 0.2mol / L NaOH: purchased from Shanghai Shisi Hewei Chemical Co., Ltd.;
[0049] 10mmol / L Tris-Acetate (pH 7.6): Tris-base was purchased from Sigma Company in the United States, and anhydrous acetic acid was purchased from Hangzhou Chemical Reagent Co., Ltd.;
[0050] Binding buffer: 10mM Tris-HCl (Tris-base was purchased from Sigma, USA; hydrochloric acid was...
Embodiment 2
[0058] Embodiment 2: detection method.
[0059] Instruments: Bio-Rad S1000 PCR instrument, Beckman Microfuge 22R desktop micro-refrigerated centrifuge, QIAGEN PyroMark Q96 ID sequencer.
[0060] (1) Extract DNA from whole blood samples:
[0061] Referring to the published literature, the corresponding commercial DNA extraction kit was used, and the genomic DNA in the blood was prepared according to the kit instructions, and used as a PCR reaction template for future use.
[0062] After the DNA is dissolved, use a Nano-Space ultraviolet spectrophotometer to measure the nucleic acid concentration. If the nucleic acid concentration is greater than 50ng / ul, it is considered qualified. Store the nucleic acid specimen in a 4°C refrigerator;
[0063] (2) using the DNA obtained in step (1) as a template, and using specific primers to perform PCR amplification;
[0064] Among them, PCR amplification adopts 50μl system, in which DNA template 2μL, 10×PCR buffer 5μL, MgCl 2 (25mmol / L) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com