Amantadine artificial antigen and preparation method and application thereof
A technology of amantadine and amantadine hydrochloride, which is applied in the field of antigen preparation and amantadine hapten, can solve the problems that the accuracy, sensitivity, and specificity of the kit cannot fully meet the detection requirements, and achieve the preparation method Simple and feasible, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, the preparation of amantadine hapten
[0028] 1. Preparation of amantadine hapten
[0029] Dissolve 0.94g of amantadine hydrochloride in 30ml of anhydrous pyridine, add 0.49g of maleic anhydride, after dissolving, add 10mg of 4-dimethylaminopyridine, reflux at 70°C for 3h, remove pyridine by rotary evaporation, add 10ml of deionized water , adjust the pH to 5.0 with glacial acetic acid, precipitate a large amount of precipitate, filter, wash the precipitate with water, and dry to obtain the hapten.
[0030] 2. Identification of amantadine hapten
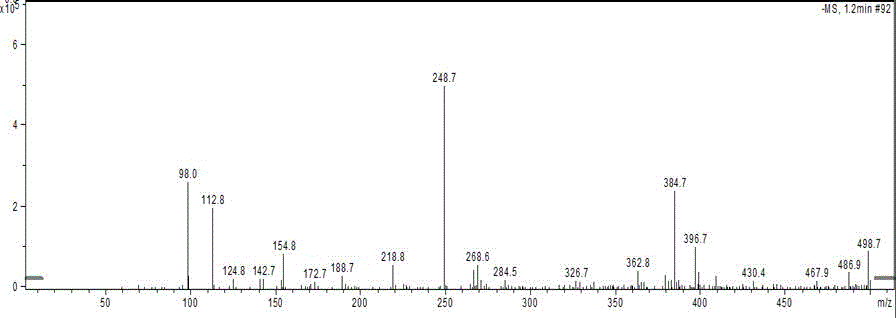
[0031] The resulting product was identified by mass spectrometry, -MS: M-1=248.7 ( figure 1 ).
[0032] The results showed that its chemical structure was as shown in Formula 1, which was the amantadine hapten.
[0033]
[0034] Formula 1.
Embodiment 2
[0035] Example 2, Preparation and Identification of Amantadine Artificial Antigen
[0036] 1. Synthesis of amantadine immune antigen
[0037] Weigh 5.57mg of hapten and dissolve it in 1ml DMF, add 4.3mg of EDC, 5.2mg of NHS, and stir at room temperature for 2h; After stirring overnight, dialyze in PBS at 4°C for 72 hours, during which time the dialysate was changed 6 times. The dialysate was passed through a filter membrane with a pore size of 0.22 μm under sterile conditions, distributed into ampoule bottles, and stored at -20°C.
[0038] Coating original was prepared in the same way.
[0039] 2. Identification of amantadine artificial antigen
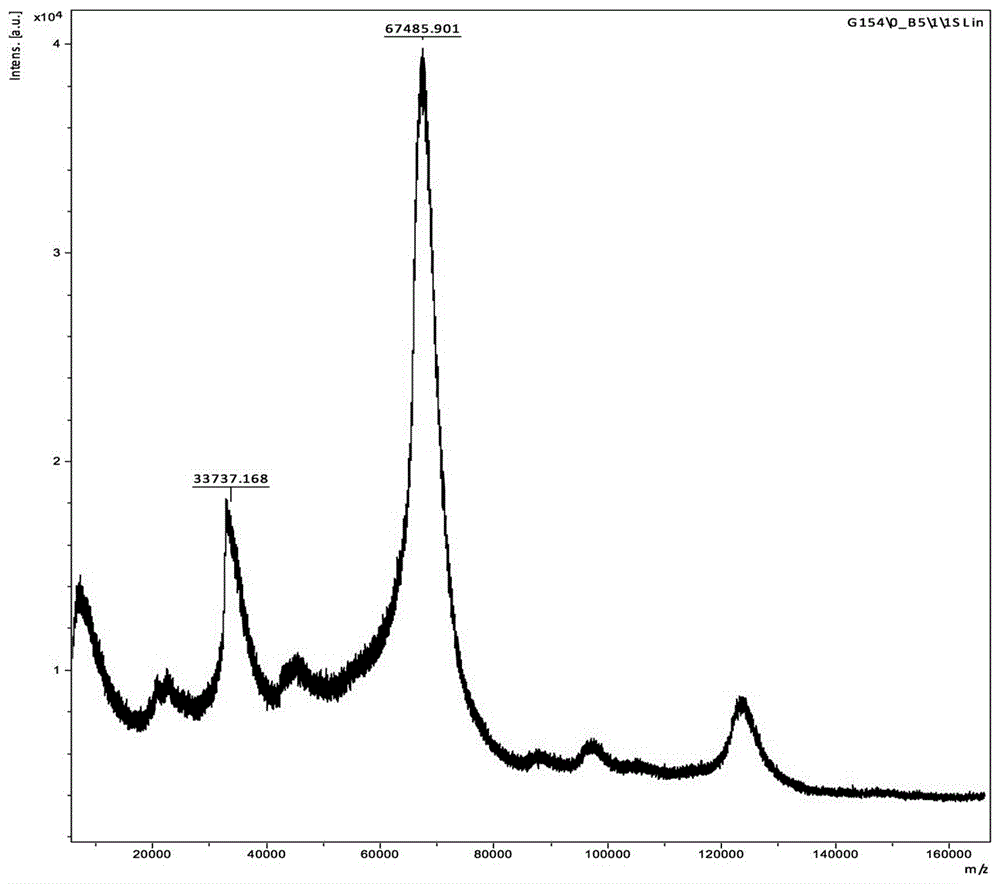
[0040] The immunogen MALDI-TOF-MS identification results showed that the coupling ratio was: R=(AMD-MH-BSA)-(BSA) / 249.7=(73828.009-67485.901) / 249.7=25.40( figure 2 and image 3 ). That is, in the immunogen, the molar ratio of the amantadine hapten (formula I) coupled to bovine serum albumin (BSA) is 25.40:1.
Embodiment 3
[0041] Example 3, Preparation and Specific Identification of Enzyme-labeled Monoclonal Antibody
[0042] 1. Preparation of amantadine monoclonal antibody
[0043] 1. Use the immunogen prepared above at 100 μg / mouse, dissolve the immunogen in normal saline and mix it with Freund’s complete adjuvant in equal volumes, inject subcutaneously on the back of the neck and immunize 6-8 week-old Balb / c female mice, after the first immunization On the 7th, 14th, and 28th days, mix the immunogen and Freund's incomplete adjuvant in equal volumes, and each booster immunization once, and 100 μg of the immune complex per mouse 3 days before the fusion, and then booster immunization without Freund's adjuvant.
[0044] 2. Proceed according to the conventional method, take the splenocytes of the immunized mice and mix them with the mouse myeloma cells (SP2 / 0) in the logarithmic growth phase, and then slowly add the preheated fusion agent (PEG4000) within 45s for fusion, Suspend evenly with HAT ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com