Cationic hyperbranched polysaccharide derivative and application of cationic hyperbranched polysaccharide derivative in phototoxicity improvement effect of hematoporphyrin photosensitizers on tumor cells
A technology of porphyrin photosensitizers and polysaccharide derivatives, which can be applied in the direction of antineoplastic drugs, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the lack of tumor tissue target Tropism, increase the toxic side effects of normal tissues, skin photosensitive period and other problems, to achieve the effect of cheap equipment and raw materials, enhanced phototoxicity, and high tumor cell lethality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of Cationic Hyperbranched Polysaccharide Derivatives and Hematoporphyrin Photosensitizer / Cation Hyperbranched Polysaccharide Derivatives Complex
[0066] 1. Preparation of cationic hyperbranched polysaccharide derivatives
[0067] (1) method
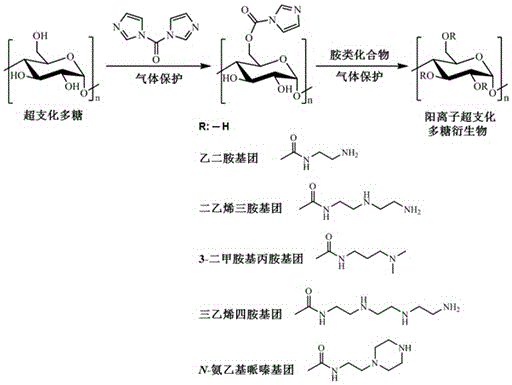
[0068] The preparation process of cationic hyperbranched polysaccharide derivatives is as attached figure 1 As shown, the specific steps are as follows:
[0069] S1. According to the ratio of polysaccharide: dehydration organic solvent = 0.1 ~ 1.0g: 10 ~ 50mL, add dehydration organic solvent to the polysaccharide, and stir at 100 ~ 500r / min at room temperature for 1 ~ 24 hours under the protection of nitrogen, argon or helium. Dissolve in 1 hour to obtain polysaccharide solution;
[0070] S2. According to N,N ’-Carbonyldiimidazole: dehydration organic solvent = 1.0~5.0g: 5~20mL ratio, to N,N Add water-removing organic solvent to dissolve in '-carbonyldiimidazole;
[0071] S3. Slowly add the solution ob...
Embodiment 2
[0091] Example 2 Preparation and Characterization of Hematoporphyrin Photosensitizer / Cation Hyperbranched Polysaccharide Derivative Complex Nanoparticles
[0092] 1. Preparation of Cationic Hyperbranched Glycogen Derivatives
[0093] (1) Weigh 0.5 g of glycogen into a dry 50 mL round bottom flask, add 10 mL of dehydrated dimethylformamide, cover with a reverse rubber stopper, and stir at room temperature for 10 hours under nitrogen protection (100r / min) dissolve.
[0094] (2) Weighing N,N Add 4.0 g of '-carbonyldiimidazole to a dry flask, add 10 mL of dehydrated dimethylformamide, cover with a glass stopper, and shake to dissolve.
[0095] (3) Slowly add the obtained solution into the glycogen solution with a syringe, and activate at room temperature for 1 hour under the protection of nitrogen (100r / min).
[0096] (4) Slowly add 12.0 mL of 3-dimethylaminopropylamine dropwise to the above reaction solution with a syringe, protect with nitrogen, and stir at room temperature f...
Embodiment 3
[0108] Example 3 Preparation of Hematoporphyrin Photosensitizer / Cation Hyperbranched Polysaccharide Derivative Complex Nanoparticles
[0109] 1. Preparation of Cationic Hyperbranched Amylopectin Derivatives
[0110] (1) Weigh 0.1g of pullulan into a dry 50 mL round bottom flask, add 20 mL of dehydrated ethyl acetate, cover with a reverse rubber stopper, and stir at room temperature for 6 hours under the protection of argon (200r / min) to dissolve .
[0111] (2) Weighing N,N Add 1.0 g of '-carbonyldiimidazole to a dry flask, add 5 mL of dehydrated ethyl acetate, cover with a glass stopper, and shake to dissolve.
[0112] (3) Slowly add the obtained solution into the pullulan solution with a syringe, and activate it at room temperature for 0.5 hours under the protection of argon (200r / min).
[0113] (4) Slowly add 10.0 mL of diethylenetriamine dropwise to the above reaction solution with a syringe, protect with argon, and stir at room temperature for 12 hours (200r / min) to rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com