High polymer CA4 bonding medicine and preparation method thereof
A technology of bonded drugs and polymers, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems affecting the tumor blood vessel inhibitory effect, and achieve short action time, Realize the effect of mass production and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
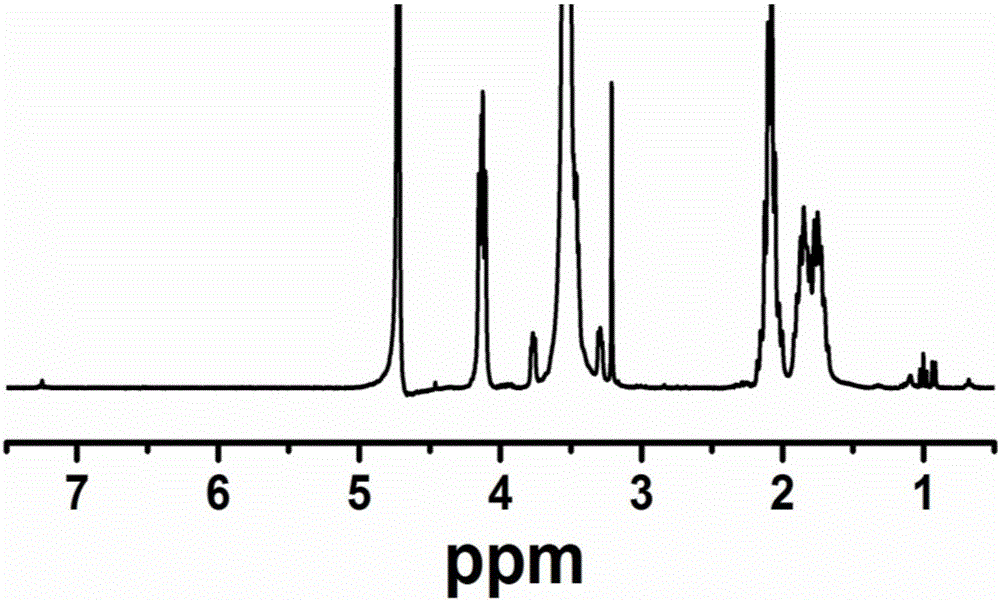
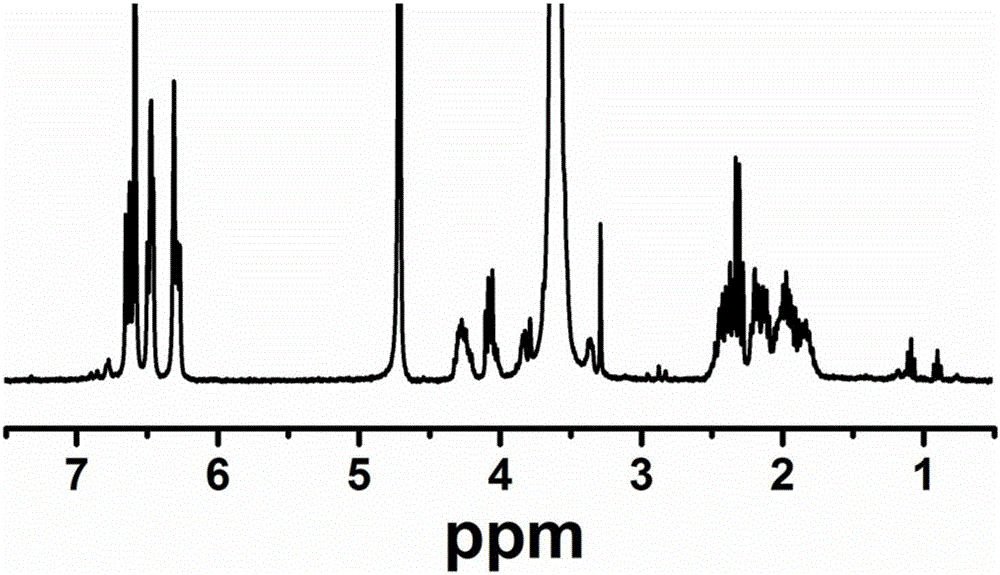
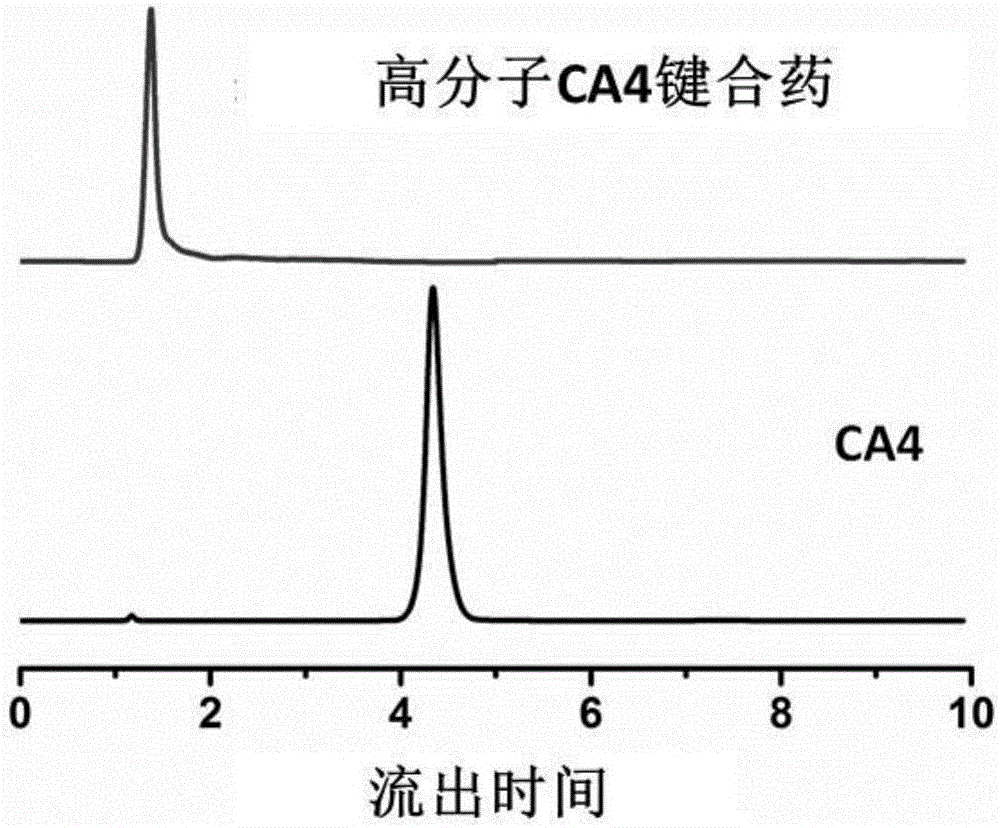
Image
Examples
preparation example Construction
[0064] The present invention also provides a preparation method of polymer CA4 bonded drug, comprising:
[0065] The copolymer having the structure of formula (II) is reacted with CA4 under the action of a condensing agent to obtain the polymer CA4 bonded drug of the structure of formula (I).
[0066]
[0067]
[0068] in,
[0069] R 1 Selected from C2-C10 straight-chain alkyl, C3-C10 branched-chain alkyl or C6-C20 aryl;
[0070] R 2 selected from a hydrogen atom or a cation;
[0071] R 3 Be selected from unsubstituted C1-C20 alkyl or substituted C1-C20 alkyl;
[0072] R 4 Alkylacyl group selected from hydrogen atom or C1-C6;
[0073] L 1 , L 2 , L 3 Independently selected from -CH 2 -or-CH 2 CH 2 -;
[0074] x, y, z are degree of aggregation, 10≤x+y+z≤5000, y>0, z>0;
[0075] n is the degree of polymerization, 10≤n≤500.
[0076] According to the present invention, the present invention reacts the copolymer with formula (II) structure and CA4 under the ef...
Embodiment 1
[0080] Dissolve 42.1 g (160.0 mmol) of γ-benzyl-L-glutamate-N-endocarboxylic anhydride monomer (BLG-NCA) in 270 mL of anhydrous N,N-dimethylformamide (DMF) After stirring and dissolving, 1.0 mL (1.0 mmol / L DMF solution) of n-hexylamine (n-HA) was added, sealed, and stirred and reacted for 72 h at a temperature of 25° C. After the reaction was finished, the obtained reaction solution was settled into 2.0L of diethyl ether, filtered and washed with diethyl ether successively, and vacuum-dried at room temperature for 24h to obtain the intermediate product poly(γ-benzyl-L-glutamate) ( PBLG).
[0081] 10.0 g of the above-prepared poly(γ-benzyl-L-glutamate) was dissolved in 100 mL of dichloroacetic acid, and under stirring conditions, 30 mL of hydrogen bromide / glacial acetic acid solution with a mass content of 33% was added, The reaction was stirred for 1 h at a temperature of 30 °C. Afterwards, the obtained reaction solution was settled into 1.0L of ether, centrifuged, and the r...
Embodiment 2
[0084] Dissolve 42.1 g (160.0 mmol) of γ-benzyl-L-glutamate-N-endocarboxylic anhydride monomer (BLG-NCA) in 270 mL of anhydrous N,N-dimethylformamide (DMF) After stirring and dissolving, 1.0 mL (1.0 mmol / L DMF solution) of n-hexylamine (n-HA) was added, sealed, and stirred and reacted for 72 h at a temperature of 25° C. Afterwards, 2.0 g (20.0 mmol) of acetic anhydride was added to the above reaction system, and the reaction was continued for 6 h. After the reaction was finished, the obtained reaction solution was settled into 2.0L of diethyl ether, filtered and washed with diethyl ether successively, and vacuum-dried at room temperature for 24h to obtain the intermediate product poly(γ-benzyl-L-glutamate) ( PBLG).
[0085] 10.0 g of the above-prepared poly(γ-benzyl-L-glutamate) was dissolved in 100 mL of dichloroacetic acid, and under stirring conditions, 30 mL of hydrogen bromide / glacial acetic acid solution with a mass content of 33% was added, The reaction was stirred fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com