Method for preparing (S)-styrene oxide through enzyme method
An epoxide and hydrolase technology, applied in fermentation and other directions, can solve the problems of low space-time yield, inability to achieve effective resolution of high-concentration substrates, etc., and achieve high catalytic efficiency, large-scale industrial application prospects, and enantiomeric purity. and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
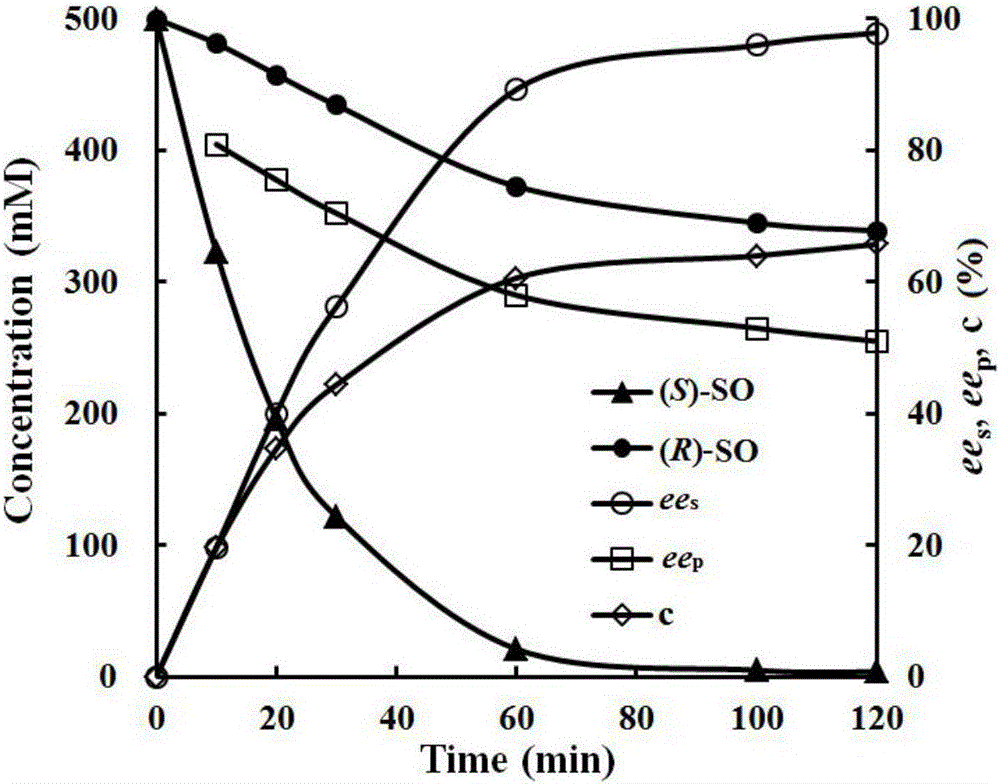
Examples
Embodiment 1
[0021] Method for measuring epoxide hydrolase enzyme activity: Add 100 μL of enzyme solution and 850 μL of potassium phosphate buffer (50 mM, pH=7.0) into a 1.5 mL EP tube, preheat at 35°C for 2 minutes; add 50 μL of 200 mM rac-SO, react After 15 minutes, add 1 mL of ethyl acetate and mix well, centrifuge at 10,000 r / min for 5 minutes, take the upper organic phase into a new EP tube, add an appropriate amount of anhydrous MgSO 4 Dried over a 0.22 μm organic membrane and analyzed by chiral gas chromatography. Chromatographic conditions: Shimadzu GC-2010GC gas chromatograph, CYCLOSIL-B chiral chromatographic column, flame ionization detector; the temperature of the injection port and the detection port is 250°C, and the temperature is programmed from 100°C to 190°C at 5°C / min ; (R)-SO and (S)-SO retention times are 6.087 and 6.187. Definition of enzyme activity unit: under the conditions of this assay, the amount of enzyme required to consume 1 μmol of styrene oxide per minute ...
Embodiment 2
[0023] Preparation of recombinant epoxide hydrolase (reAuEH2): the nucleic acid sequence and amino acid sequence of AuEH2 and the construction of engineering bacteria E.coli / (reAuEH2) expressing reAuEH2 refer to the invention patent application with publication number CN102994470A, pick E. The coli / (reAuEH2) single colony was cultured in LB medium at 37°C for 12h, and then transferred into fresh 100mL LB medium with an inoculum size of 2% (v / v), and cultured at 37°C for 2h, Add 0.2mM IPTG inducer and incubate at 28°C for 8h to induce the high expression of reAuEH2. The recombinant cells were collected by centrifugation at 8000rpm, suspended by adding phosphate buffer (50mM, pH7.0), and the supernatant was collected by centrifugation after sonication. The reAuEH2 enzyme solution obtained by concentrating in an ultrafiltration centrifuge tube was determined to have a protein content of 40 mg / mL and a specific enzyme activity of 3.8 U / mg.
Embodiment 3
[0024] The influence of embodiment 3 organic solvents on the activity of epoxide hydrolase
[0025] In a 1mL reaction system, containing 20mM rac-SO and 100μL appropriately diluted reAuEH2 enzyme solution and phosphate buffer, and adding 10%, 15%, 25% and 35% (v / v) water-soluble organic solvent methanol , DMSO, DMF and isopropanol were reacted at 35°C for 15 minutes to measure the effect of water-soluble organic solvents on reAuEH2 enzyme activity, and the reaction system without adding any organic solvent was used as a control. When the measured water-soluble organic solvent addition amount is 10%, reAuEH2 retains >95% of the enzyme activity, when the addition amount of DMSO is 25%, the enzyme activity of reAuEH2 retains 50%, and the addition amount of methanol, DMF and isopropanol is 25% % when reAuEH2 only retains <30% enzymatic activity.
[0026] In addition, reAuEH2 enzyme liquid and ethyl acetate, butyl acetate, dichloromethane, chloroform, toluene, isobutanol, isoamyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com