Artificial blood vessel and preparation method thereof
A technology of artificial blood vessels and solutions, applied in blood vessels, devices of human tubular structures, anticoagulant treatment, etc., can solve the problems of inability to prepare implantable artificial blood vessel repair materials, etc., and achieve improved mechanical strength and excellent anticoagulation The effect of the function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The invention provides a method for preparing the artificial blood vessel, which is characterized in that the method comprises the following steps:
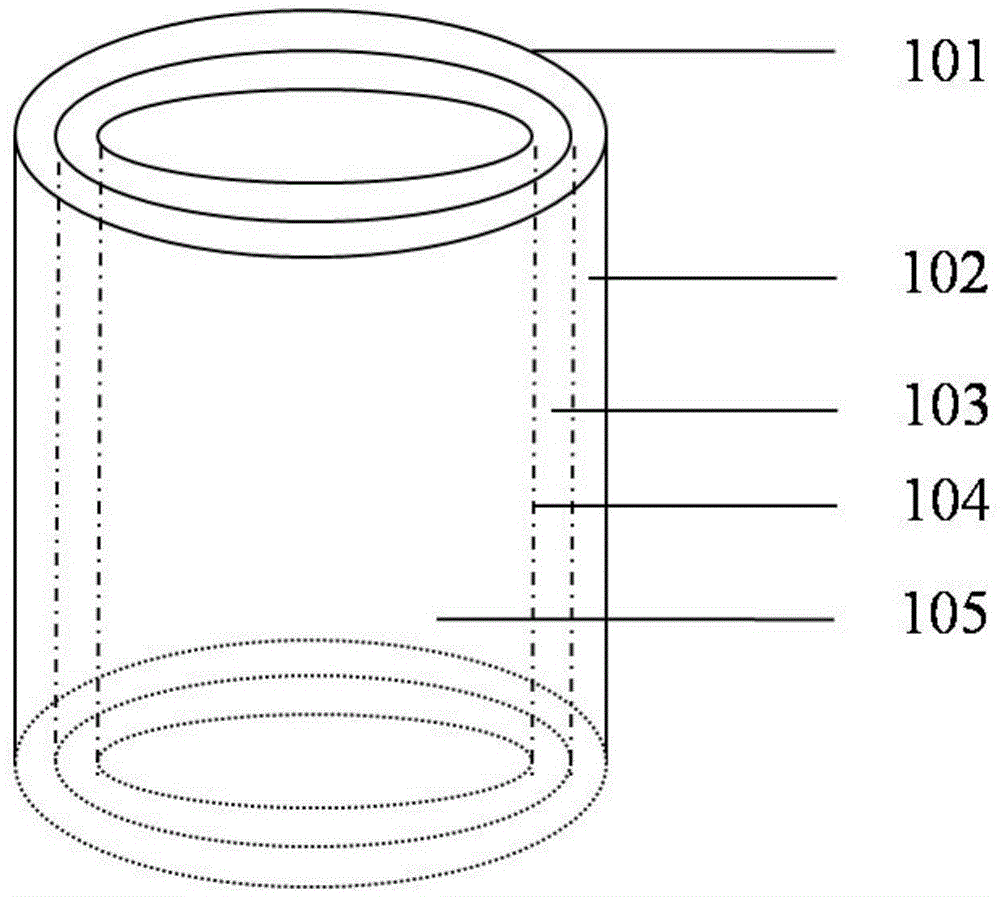
[0035] 1) First prepare the natural macromolecule solution with a mass percentage of 0.1-20%, and add an anticoagulant factor with a mass percentage of 0.01-1% in the natural macromolecular solution; Add endothelial cells, smooth muscle cells, fibroblasts, stem cells, or a mixture of stem cells and growth factors to prepare a natural polymer solution containing different cells, wherein the density of cells in the natural polymer solution is 1×10 2-7 a / mL;
[0036] 2) Prepare a series of hollow cylindrical molds with different diameters, put a second hollow cylindrical mold with a larger diameter on the outside of the first hollow cylindrical mold, leave a gap between the two molds, and use a dropper or syringe to put the endothelial cells, or The mixture of stem cells and endothelial cells, or the natural polymer solution...
Embodiment 1
[0047] Example 1: A small-diameter blood vessel and its preparation method
[0048] a) Prepare 0.1% sodium alginate and 20% gelatin solutions by mass percentage, mix the sodium alginate and gelatin solutions uniformly at a volume ratio of 1:1, add 1% anticoagulant heparin and 10% Cell cryopreservation factor dimethyl sulfoxide; mix endothelial cells, smooth muscle cells, and fibroblasts with the mixture, and the density of cells in the mixture of sodium alginate and gelatin is 1×10 2 a / mL;
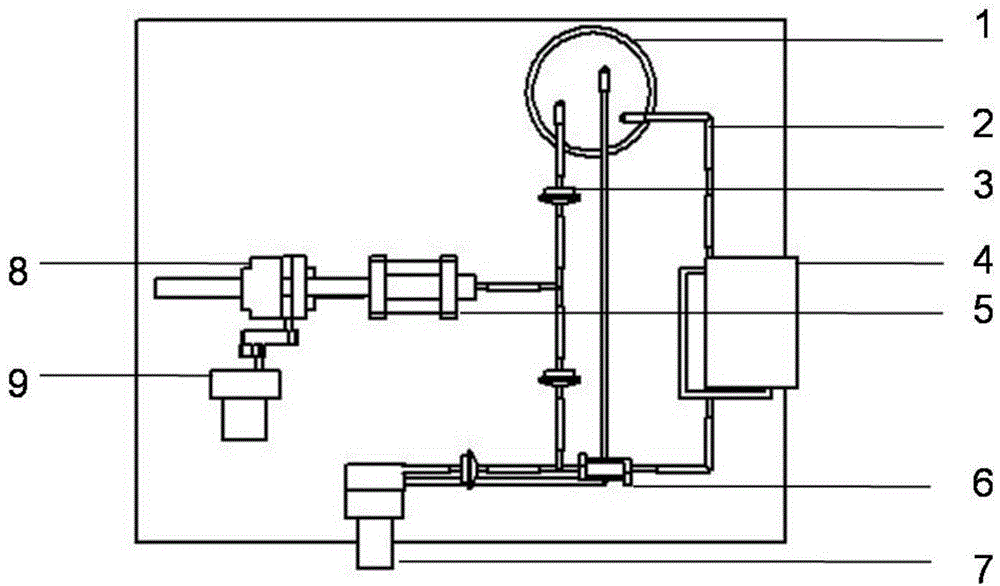
[0049] b) Load various cell-containing solutions in step a) into the nozzle assembly of the 3D printing device, and print layers with a lumen diameter of 0.1mm, which are endothelial cell layer, smooth muscle cell layer and fibroblast cell layer from inside to outside. Hollow cylinder; use 1% calcium chloride solution to cross-link sodium alginate molecules to stabilize the three-dimensional structure;
[0050] c) Spraying a layer of polyethylene solution with a mass volume percentage of 3...
Embodiment 2
[0052] Example 2: A large-diameter blood vessel and its preparation method
[0053] a) Prepare a series of hollow cylindrical molds with a diameter greater than 6mm, respectively prepare fibrinogen solutions containing bone marrow stem cells, endothelial cells and fibroblasts, wherein the mass percentage of the fibrinogen solution is 5%, and the amount of cells in the fibrinogen solution Density is 1×10 7 cells / mL, add 1% smooth muscle cell growth factor, 1% anticoagulant heparin and 10% cell cryopreservation factor dimethyl sulfoxide to the bone marrow stem cell solution;
[0054] b) Put a second hollow cylinder mold with a larger diameter on a first hollow cylinder mold with a smaller diameter, leave a gap in the middle of the two molds, inject the fibrinogen solution containing endothelial cells and heparin into the two molds with a dropper In the gap between the moulds, 10% thrombin is used to polymerize the fibrinogen to form an endothelial cell layer;
[0055] c) Remov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com