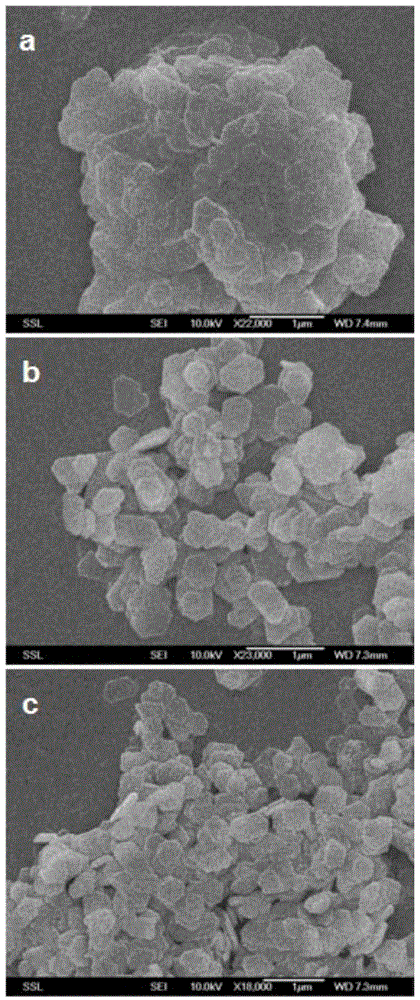
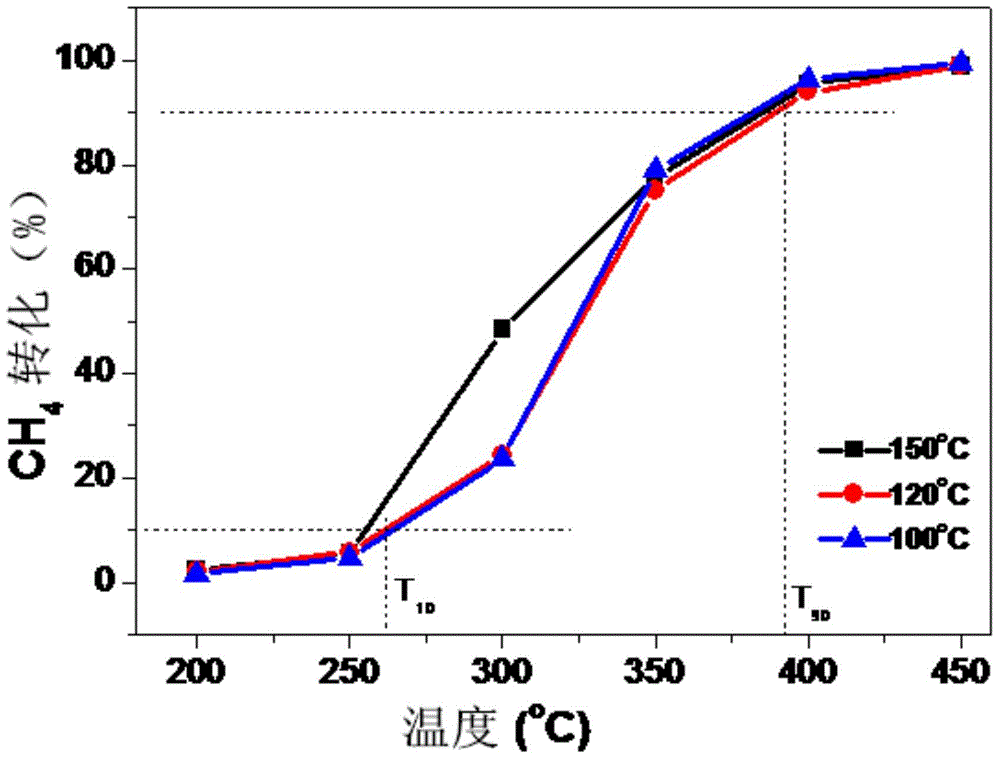
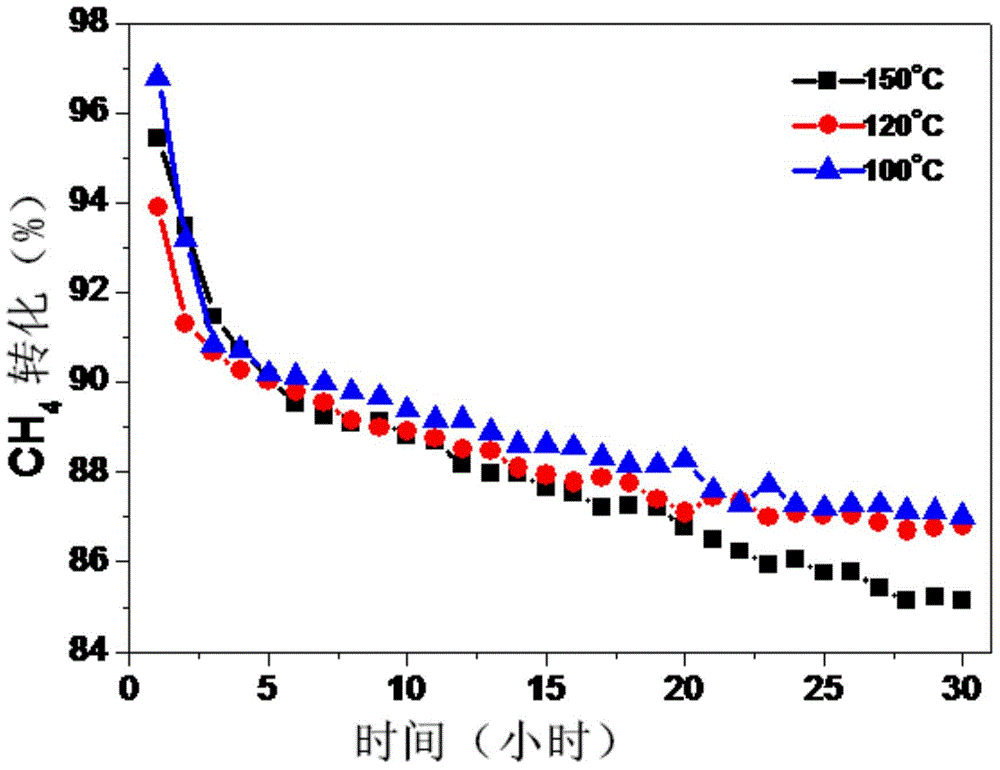
Preparation method of Co3O4 catalyst, and application of the catalyst in catalytic combustion of methane
A technology of tricobalt tetroxide catalyst and tricobalt oxide catalyst, which is applied in the direction of cobalt oxide/cobalt hydroxide, fuel, fuel additive, etc., can solve the problems of heavy metal pollution, harsh preparation process, unsuitable for mass production and promotion, and achieve low light-off temperature , simple process, excellent low temperature activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This example is an example of the preparation method of the methane combustion catalyst of the present invention.
[0032] a. Dissolution: Dissolve 3.10 g (about 0.0175 mol) of cobalt acetate in a mixed solution of 10 mL (about 0.1799 mol) of ethylene glycol and 30 mL (about 1.6667 mol) of water to form solution A (wherein ethylene glycol, water and The ratio of the amount of cobalt acetate is about 10.79:100:1.05); 2.0 grams of sodium hydroxide are dissolved in another part of 10mL ethylene glycol and 30mL water mixed solution to form solution B (wherein ethylene glycol, water The ratio of the amount of substance to sodium hydroxide is about 10.79:100:3); the cobalt acetate and sodium hydroxide in solution A and solution B are fully dissolved by electromagnetic stirring respectively to form a uniform solution.
[0033] b. Mixing: Mix solution A prepared in step a above with solution B, stir with a glass rod for 10 minutes, then pour into a hydrothermal reaction kettle....
Embodiment 2
[0038] This example is an example of the preparation method of the methane combustion catalyst of the present invention.
[0039]a. Dissolution: Dissolve 3.10 g (about 0.0175 mol) of cobalt acetate in a mixed solution of 10 mL (about 0.1799 mol) of ethylene glycol and 30 mL (about 1.6667 mol) of water to form solution A (wherein ethylene glycol, water and The ratio of the amount of cobalt acetate is about 10.79:100:1.05); 2.0 grams of sodium hydroxide are dissolved in another part of 10mL ethylene glycol and 30mL water mixed solution to form solution B (wherein ethylene glycol, water The ratio of the amount of substance to sodium hydroxide is about 10.79:100:3); the cobalt acetate and sodium hydroxide in solution A and solution B are fully dissolved by electromagnetic stirring respectively to form a uniform solution.
[0040] b. Mixing: Mix solution A prepared in step a above with solution B, stir with a glass rod for 10 minutes, then pour into a hydrothermal reaction kettle. ...
Embodiment 3
[0045] This example is an example of the preparation method of the methane combustion catalyst of the present invention.
[0046] a. Dissolution: Dissolve 3.10 g (about 0.0175 mol) of cobalt acetate in a mixed solution of 10 mL (about 0.1799 mol) of ethylene glycol and 30 mL (about 1.6667 mol) of water to form solution A (wherein ethylene glycol, water and The ratio of the amount of cobalt acetate is about 10.79:100:1.05); 2.0 grams of sodium hydroxide are dissolved in another part of 10mL ethylene glycol and 30mL water mixed solution to form solution B (wherein ethylene glycol, water The ratio of the amount of substance to sodium hydroxide is about 10.79:100:3); the cobalt acetate and sodium hydroxide in solution A and solution B are fully dissolved by electromagnetic stirring respectively to form a uniform solution.
[0047] b. Mixing: Mix solution A prepared in step a above with solution B, stir with a glass rod for 10 minutes, then pour into a hydrothermal reaction kettle....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com