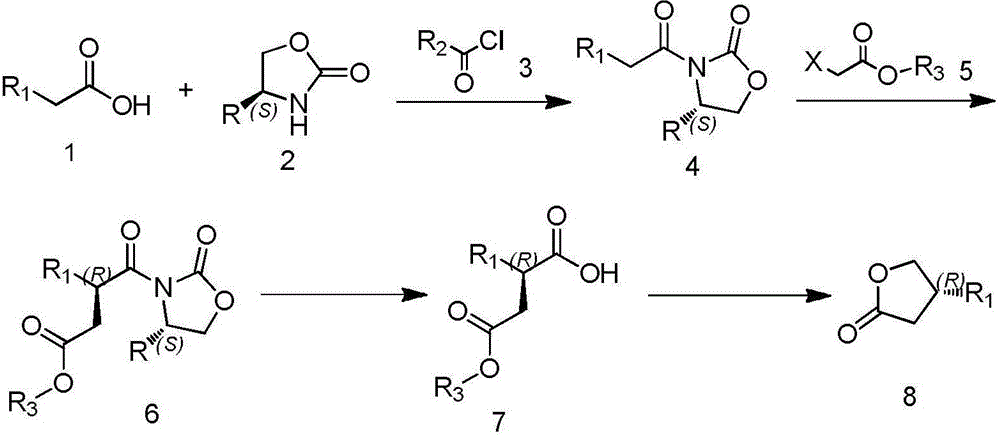
Method for preparing chiral 4-substituted dihydrofuran-2(3H)-ketone
A technology of dihydrofuran and substituents, which is applied in the field of preparation of chiral 4-substituent dihydrofuran-2-one, can solve problems such as difficult industrial production, complex process, and difficult process, and achieve easy industrial production, The effect of simple preparation process and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
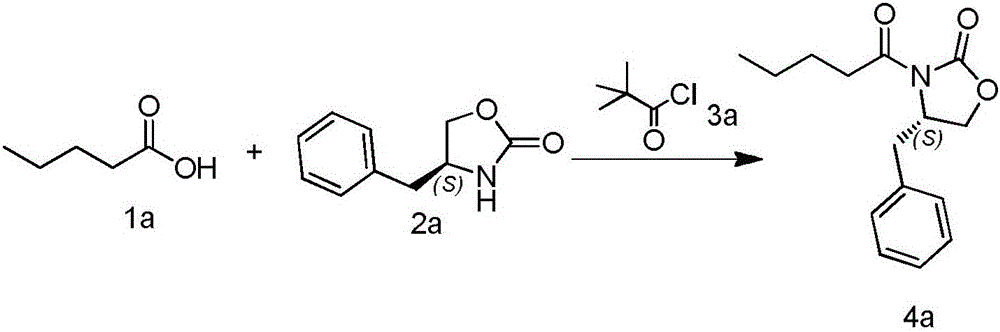
[0045] Synthesis of (S)-4-benzyl-3-pentanoyloxazolidin-2-one (compound 4a)
[0046]
[0047]Add 1 L of tetrahydrofuran, 70.47 g of valeric acid (compound 1a), and 155.83 g of triethylamine into the reaction flask, and cool to -10°C under nitrogen protection. 83.49 g of pivaloyl chloride (compound 3a) was slowly added dropwise. Keep stirring at -10°C for 0.5 hour, and add 31.37 g of anhydrous lithium chloride. A tetrahydrofuran solution of 100.89 g of (S)-4-benzyloxazolidin-2-one (compound 2a) was slowly added dropwise. The temperature was naturally raised to room temperature (20-25)°C, and stirring was continued for 16 hours. After filtration, 1.2 L of 10% potassium carbonate was added to the filtrate, stirred for 10 minutes, THF was distilled off under reduced pressure, and extracted with tertiary methyl ether. The tertiary methyl ether layer was washed successively with 500 mL of 5% dilute hydrochloric acid and 500 mL of saturated sodium bicarbonate. It was dried, fil...
Embodiment 2
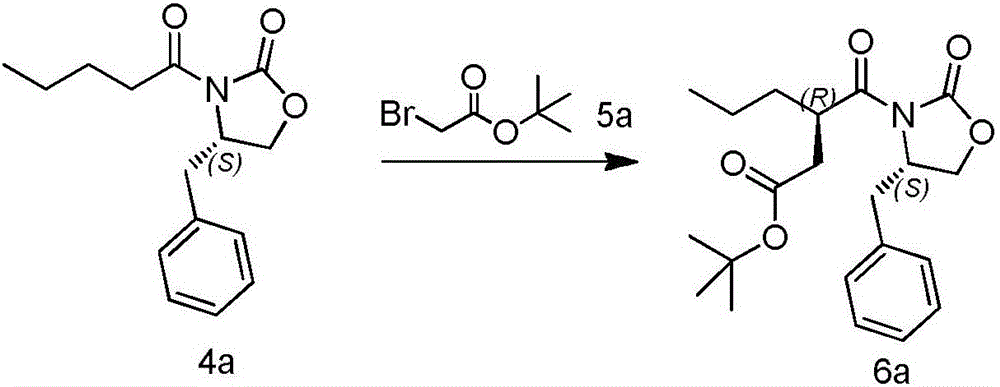
[0049] Synthesis of tert-butyl (R)-3–((S)--4-benzyl-2-oxazolidinone-3-carbonyl)hexanoate (compound 6a)
[0050]
[0051] 140 g of (S)-4-benzyl-3-pentanoyloxazolidin-2-one (compound 4a) was dissolved in 1 L of tetrahydrofuran, protected by nitrogen, and cooled to -70°C to -65°C. 290 mL of 2 mol / L sodium bis(trimethylsilyl)amide (NaHMDS) was added dropwise, and the temperature was controlled below -65°C. After the dropwise addition, keep warm for 0.5 hours. 134.4 g of tert-butyl bromoacetate (compound 5a) were added dropwise. After the dropwise addition, the temperature was naturally raised to room temperature (20-25° C.), and stirring was continued for 10 minutes. Add 500 mL of saturated ammonium chloride to quench. The reaction solution was extracted with tertiary methyl ether (800 mL×3), dried, filtered, and concentrated to dryness under reduced pressure. Then recrystallize (methyl tert-ether:petroleum ether=1:5) to obtain 143 grams of (R)-3-((S)--4-benzyl-2-oxazolidin...
Embodiment 3
[0053] Synthesis of (R)-2-(2-(tert-butoxy)-2-oxoethyl)pentanoic acid (Compound 7a)
[0054]
[0055] 318 grams of (R)-3-((S)--4-benzyl-2-oxazolidinone-3-carbonyl) tert-butyl hexanoate (compound 6a) was dissolved in 2.25 L tetrahydrofuran and 0.75 L water In the mixed solvent, stir in an ice bath. 428 grams of hydrogen peroxide was added dropwise while keeping below 5°C. 70.4 g of lithium hydroxide hydrate was added. Stir at room temperature (20-25° C.) for 15 hours. Add water for dilution, and add sodium sulfite solution (476 g of sodium sulfite in 2 L of water) dropwise under ice-cooling, keeping the temperature below 10°C. Add 1.5L of ethyl acetate, stir and separate the layers, and wash the water layer once with 800mL of ethyl acetate. Add 400 g of citric acid to adjust the aqueous layer to pH = 3, and extract with ethyl acetate (600 mL×3). It was dried, filtered, and concentrated to obtain 165 g of oily (R)-2-(2-(tert-butoxy)-2-oxoethyl)pentanoic acid (compound 7a,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com