Composite-structure anti-inflammatory medicine-carrying fiber for transdermal drug delivery
A composite structure, anti-inflammatory drug technology, applied in the direction of anti-inflammatory agent, drug combination, fiber treatment, etc., can solve the problems of poor strength, pure bacterial cellulose is not suitable for drug-carrying system, and drugs are prone to burst release phenomenon, etc. Expand the scope of application and reduce the effect of secondary damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
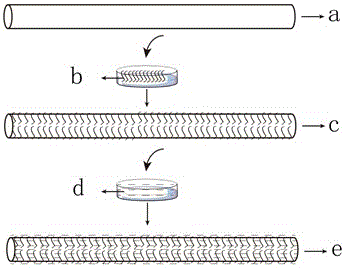
[0026] An anti-inflammatory drug-loaded fiber with a composite structure for transdermal administration and its preparation method:
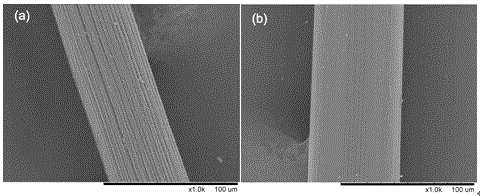
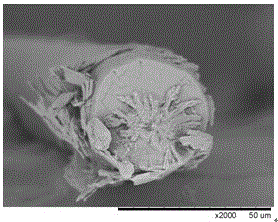
[0027] The first step: the preparation of bacterial cellulose fibers. With N,N-dimethylacetamide / lithium chloride (DMAc / LiCl), zinc chloride (ZnCl 2 ), ionic liquid, N-methylmorpholine aqueous solution (NMMO) any one of the solvents, the bacterial cellulose solid content of 1% (w / w) spinning solution prepared at room temperature, wet spinning, dry spinning Bacterial cellulose fibers were prepared by conventional spinning or dry-jet wet spinning.
[0028] The second step: drug-loading finishing of bacterial cellulose fibers. The drugs are anti-inflammatory drugs, including aspirin, paracetamol, indomethacin, naproxen, naproxen, diclofenac, ketoprofen, ibuprofen, nimesulide, rofecoxib , celecoxib, etc., or a combination thereof, anti-inflammatory drugs and transdermal enhancers (one of azone, oleic acid, N-methyl-2-pyrrolidone, propylene glycol...
Embodiment 2
[0032] In this example, the mass concentration of bacterial cellulose A spinning solution is 1.5%, the dissolved concentration of anti-inflammatory drug B is 60 g / L (w / w), and the dissolved concentration of transdermal enhancer C is 60 g / L (w / w). / w), polyacrylonitrile D is a dilute solution with a solution mass concentration of 1% (w / w), and the other methods are the same as in Example 1.
Embodiment 3
[0034] The mass concentration of bacterial cellulose A spinning solution was 2%, the dissolved concentration of anti-inflammatory drug B was 70 g / L (w / w), and the dissolved concentration of transdermal enhancer C was 70 g / L (w / w). Acrylonitrile D solution is a dilute solution with a mass concentration of 3% (w / w), and other methods are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com