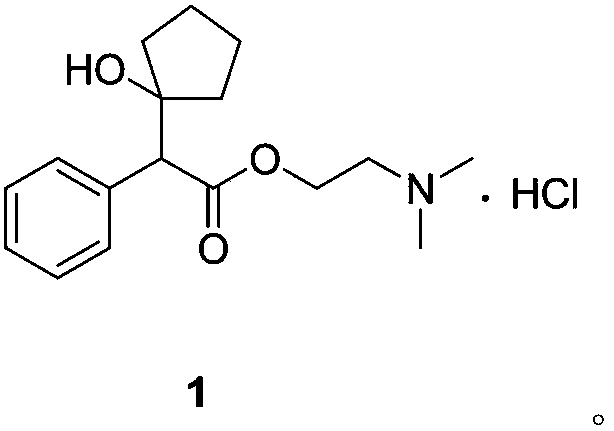
A kind of preparation method of cyclopentolate hydrochloride
A technology of cyclopentolate hydrochloride and hydrochloric acid, which is applied in the field of preparation of cyclopentolate hydrochloride, can solve the problems of high labor protection requirements for workers, poor reproducibility of the preparation process, unfavorable large-scale preparation, etc., to achieve environmental protection and The effect of safe production in the workshop, avoiding the use of flammable, explosive and toxic reagents, and reducing labor protection requirements for workers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
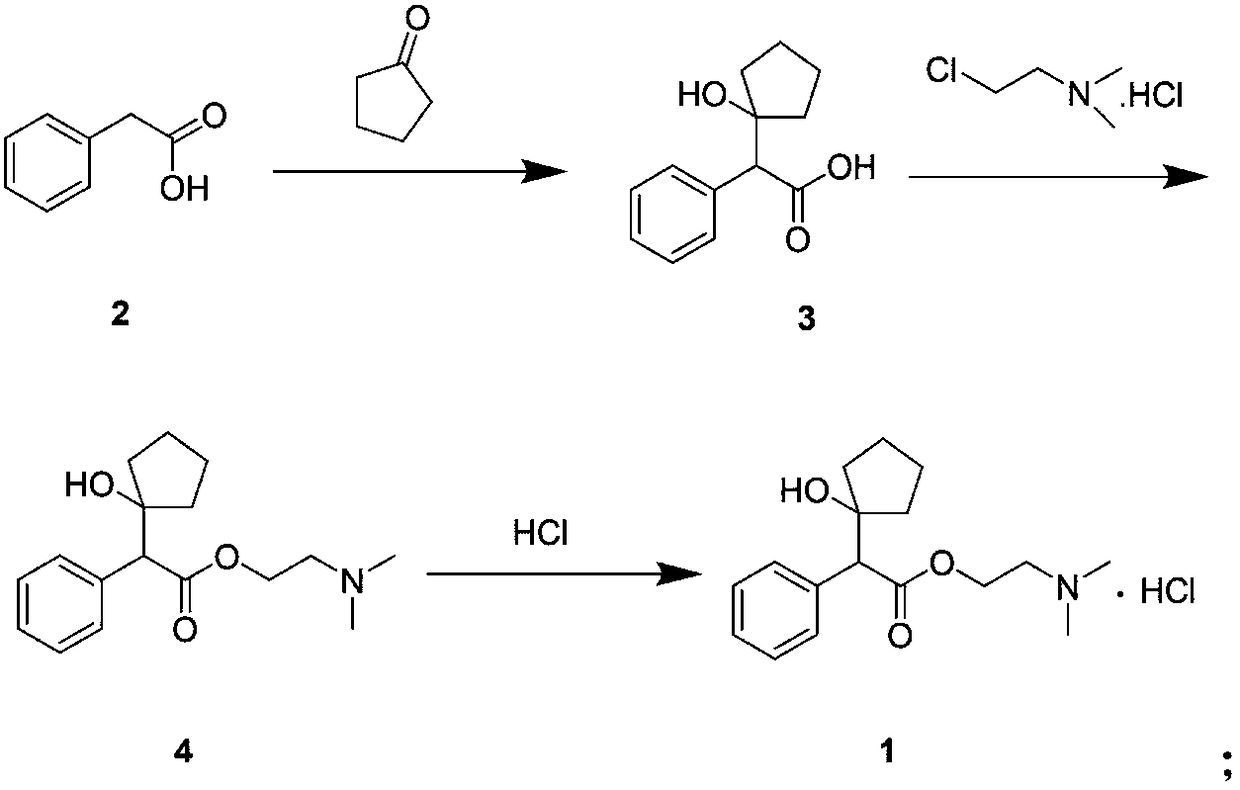
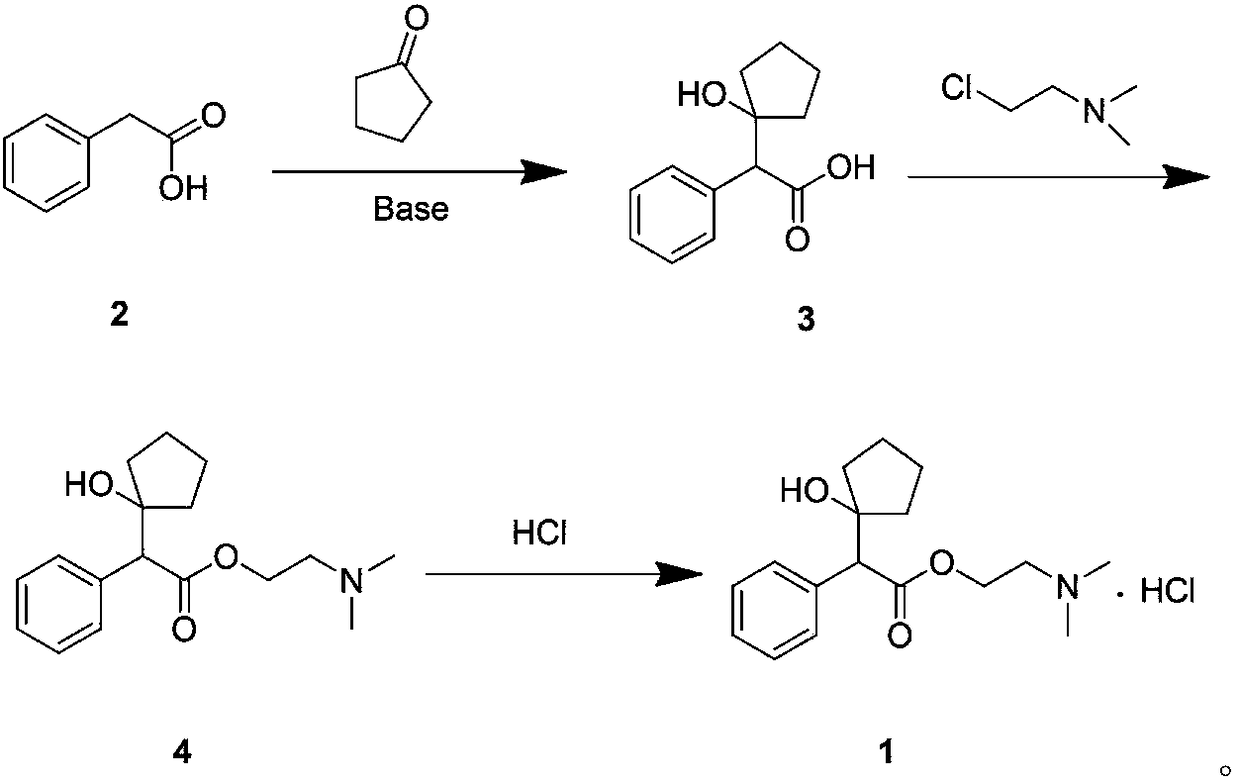
[0035] (1) Synthesis of intermediate 3
[0036] N 2 Under protection, phenylacetic acid (7.0 g) and acetonitrile (250 mL) were sequentially added into a 500 mL reaction flask, stirred to dissolve, cooled to 0° C., potassium tert-butoxide (15.0 g) was added in batches, and reacted at room temperature for 1 h. Then, the temperature of the system was lowered to 0° C., and an acetonitrile solution (140 mL) of cyclopentanone (9.0 mL) was slowly added dropwise. After dropping, slowly rise to room temperature and continue to react for 5h. After the reaction was detected by TLC, 200 mL of saturated ammonium chloride solution was added to the system, stirred for 20 min, and ethyl acetate (30 mL×2) was added to the residual liquid for extraction. The aqueous layer was slowly adjusted to pH 2 with 0.1 mol / L hydrochloric acid, added dichloromethane (50 mL×2) for extraction, the organic layer was washed with water, dried, and concentrated under reduced pressure to obtain intermediate 3 (...
Embodiment 2
[0042] (1) Synthesis of intermediate 3
[0043] N 2Under protection, phenylacetic acid (3.5g) and dioxane (120mL) were sequentially added into a 250mL reaction flask, stirred to dissolve, cooled to 0°C, sodium hydride (3.0g) was added in batches, and reacted at room temperature for 1h. The system was cooled down to -20°C, and a solution of cyclopentanone (5.5 mL) in dioxane (90 mL) was slowly added dropwise. After dropping, slowly rise to room temperature and continue to react for 5h. After the reaction was detected by TLC, 100 mL of saturated ammonium chloride solution was added to the system, stirred for 20 min, and ethyl acetate (20 mL×2) was added to the residual liquid for extraction. The aqueous layer was slowly adjusted to pH 6 with 10mol / L dilute sulfuric acid, extracted with dichloromethane (30mL×2), the organic layer was washed with water, dried, and concentrated under reduced pressure to obtain intermediate 3 (3.6g, yield 63%), purity 93.80% (HPLC normalization m...
Embodiment 3
[0049] (1) Synthesis of intermediate 3
[0050] Phenylacetic acid (7.0 g) and tetrahydrofuran (250 mL) were sequentially added into a 500 mL reaction flask, stirred to dissolve, and cooled to 0°C. N 2 Under replacement conditions, 2 mol / L lithium diisopropylamide tetrahydrofuran solution (36 mL) was added in batches and reacted at room temperature for 1 h. The system was cooled down to -60°C, and a tetrahydrofuran solution (140 mL) of cyclopentanone (5.5 mL) was slowly added dropwise. After dropping, slowly rise to room temperature and continue to react for 10h. After the reaction was detected by TLC, 200 mL of saturated ammonium chloride solution was added to the system, stirred for 20 min, and ethyl acetate (30 mL×2) was added to the residual liquid for extraction. The aqueous layer was slowly adjusted to pH 5 with 6mol / L acetic acid, extracted with dichloromethane (50mL×2), the organic layer was washed with water, dried, and concentrated under reduced pressure to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com