Reductively-responsive paclitaxel prodrug and preparation method for nano-micelle carrier
A nanomicelle, paclitaxel technology, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of limited drug encapsulation efficiency and drug loading, and achieve drug release. Significantly rapid, increased drug loading, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
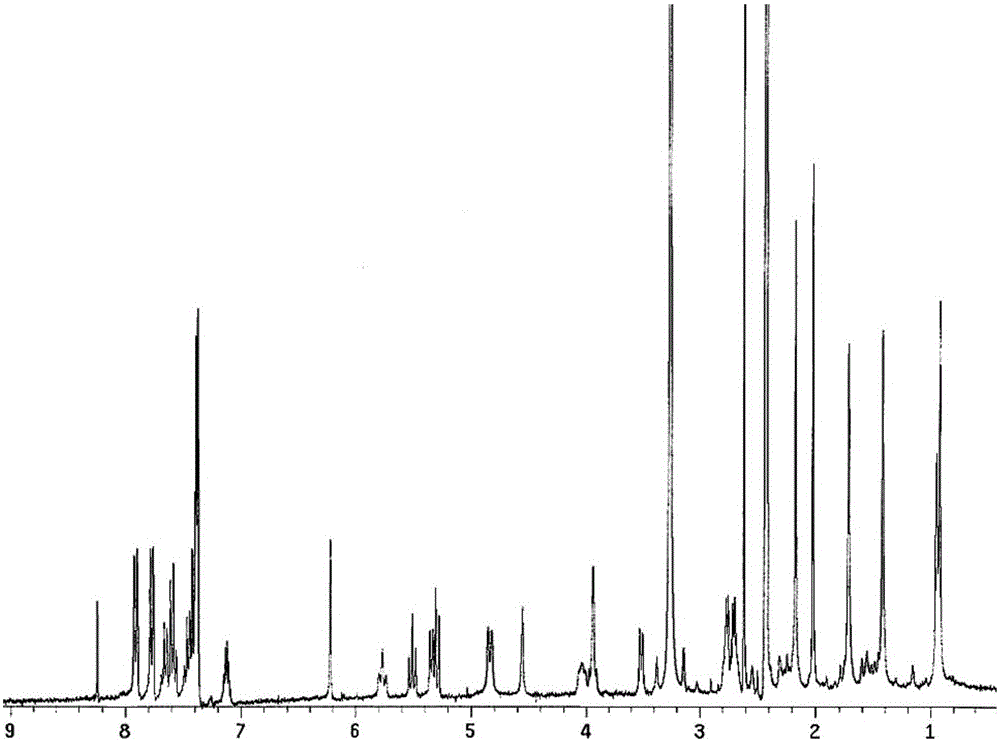
[0026] According to the following synthetic route, 11.7 mg of dithioglycolic acid, 5 mL of dichloromethane, and 55.5 mg of HBTU (O-benzotriazole-tetramethylurea) were added to a 50-ml round-bottomed flask, stirred magnetically, and in an ice-water bath. Fluorophosphate 0.146mmol), 58μL DIPEA (N,N-diisopropylethylamine, 0.351mmol) was slowly added dropwise, after 20 minutes of reaction, 100mg PTX (0.117mmol) was added, nitrogen protection, dark, stirred at room temperature for 20 After hours, the reaction was monitored by thin layer chromatography (TLC:chloroform:methanol=15:1, Rf=0.6). 55 ml of dichloromethane was added to the reaction solution, washed with 10 mL of 5% aqueous sodium bicarbonate solution, 10 mL of 5% glacial acetic acid, and 10 mL of water. The organic layer was dried over anhydrous sodium sulfate, filtered with suction, and concentrated under reduced pressure to obtain the crude product . The reduction-sensitive paclitaxel prodrug PTX-SS-PTX was obtained aft...
Embodiment 2
[0028] According to the synthetic route, 20 mg of dithiodibenzoic acid and 60 mg of HBTU were dissolved in 6 ml of dichloromethane, and 60 μL of DIPEA was slowly added dropwise in an ice bath. After 20 minutes of reaction, 90 mg of docetaxel was added, nitrogen protection, and dark , stirred at room temperature for 20 hours, and monitored the reaction by thin layer chromatography (TLC:chloroform:methanol=15:1). 55 ml of dichloromethane was added to the reaction solution, washed with 10 mL of 5% aqueous sodium bicarbonate solution, 10 mL of 5% glacial acetic acid, and 10 mL of water. The organic layer was dried over anhydrous sodium sulfate, filtered with suction, and concentrated under reduced pressure to obtain the crude product . The reduction-sensitive docetaxel prodrug PTX-SS-PTX was obtained after purification by silica gel column chromatography with chloroform:methanol=100:1.
Embodiment 3
[0029] Example 3: Preparation of Nanomicellar Carrier
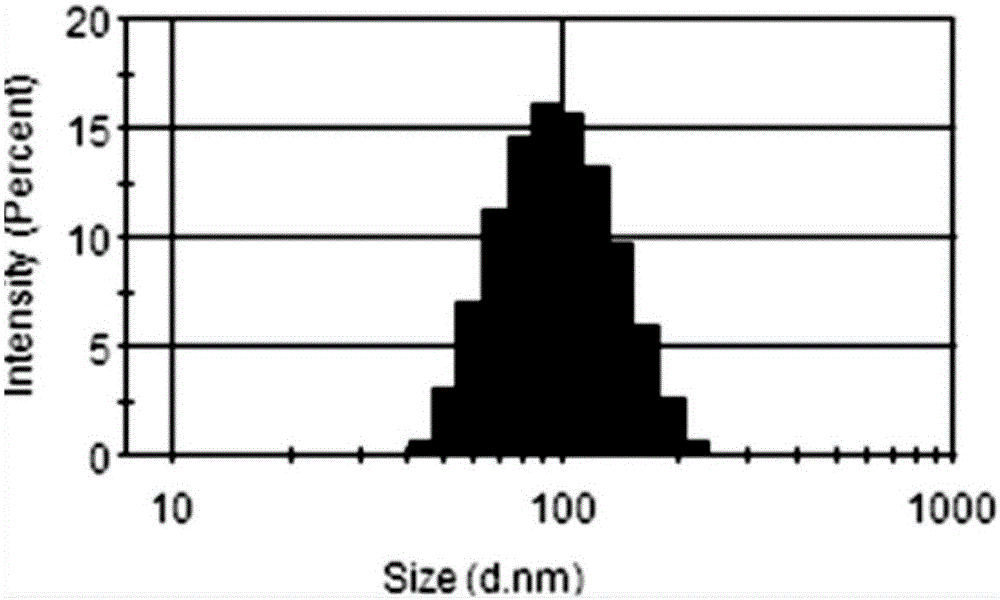
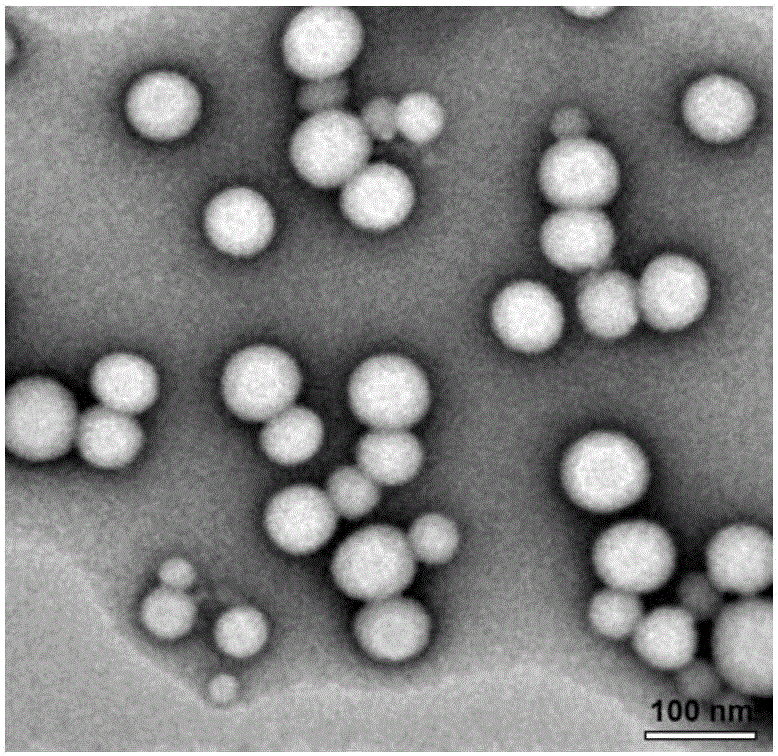
[0030] Using the solvent evaporation method, 30mg PTX-SS-PTX and 10mg MePEG-DSPE were dissolved in 10mL of absolute ethanol, slowly added dropwise to 100mL of deionized water, stirred, and the ethanol was removed by rotary evaporation at 65 °C, respectively, after passing 0.8 μm and 0.45 μm The microporous filter membrane was obtained to obtain the nanomicelle carrier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com