Nitrogen functional carbon material loaded with transition metal chalcogenide as well as preparation and application of nitrogen functional carbon material
A technology of transition metal chalcogenides and chalcogenides, applied in chemical instruments and methods, physical/chemical process catalysts, electrolysis processes, etc., can solve the problems of hydrogen production efficiency gap and achieve high efficiency and excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
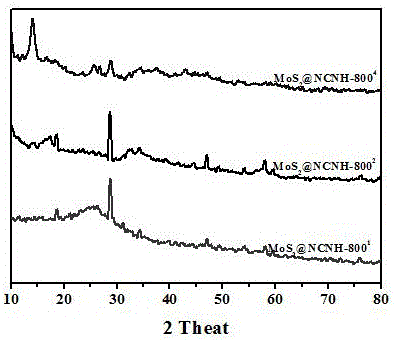
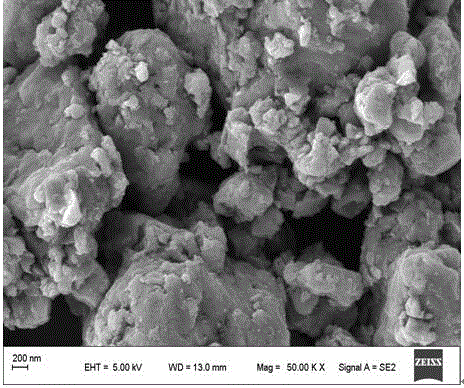
[0029] Example 1. Nitrogen-functionalized carbon material MoS 2 @NCNH-800 2 preparation of
[0030] (1) Preparation of nitrogen-doped zeolite imidazolate framework material: disperse 4g of 2-methylimidazole in 46mL of ammonia solution as solution A; disperse 4g of zinc acetate dihydrate in 26mL of deionized water as solution B, After mixing the above two solutions, stir at room temperature for 3 hours, collect the product by filtration, wash the product with deionized water until neutral, soak it in chloroform overnight, collect it by centrifugation, and dry it in vacuum at 100°C overnight to obtain nitrogen-doped zeolite imidazole Ester skeleton structure material.
[0031](2) Preparation of nitrogen-functionalized carbon material: Weigh 4g of zinc chloride and dissolve it completely in 25mL of ethanol, then disperse the nitrogen-doped zeolite imidazolate framework material prepared above in it, and stir in an oil bath at 80°C Until evaporated to dryness, under the protect...
Embodiment 2
[0034] Example 2. Nitrogen-functionalized carbon material CoSe 2 @NCNH-800 2 preparation of
[0035] (1) Preparation of nitrogen-doped zeolite imidazolate framework material: same as Example 1;
[0036] (2) Preparation of nitrogen functionalized carbon material: same as Example 1;
[0037] (3) Nitrogen-functionalized carbon material CoSe 2 @NCNH-800 2 Preparation of nitrogen-functionalized carbon material: Disperse 170 mg of nitrogen-functionalized carbon material in 180 mL of absolute ethanol, and stir for 10 to 60 minutes to obtain a suspension; add 51 mg of cobalt chloride, 88 mg of selenium dioxide, and 2.1 g of sodium acetate to the above suspension, Stir for 12-24 hours under gas protection; ultrasonically disperse sodium borohydride in absolute ethanol, add sodium borohydride ethanol solution dropwise to the above solution, stir for 1-24 hours, collect the product by centrifugation, and wash with absolute ethanol Multiple times, dry at 60°C for 24~64 hours. Grind ...
Embodiment 3
[0039] Example 3. Nitrogen functionalized carbon material CoS 2 @NCNH-800 2 preparation of
[0040] (1) Preparation of nitrogen-doped zeolite imidazolate framework material: same as Example 1;
[0041] (2) Preparation of nitrogen functionalized carbon material: same as Example 1;
[0042] (3) Nitrogen-functionalized carbon material CoS 2 @NCNH-800 2 Preparation: Disperse 700mg of cobalt chloride and 1.5g of thiourea in 80mL of deionized water to obtain a clear solution, then disperse 50mg of nitrogen-functionalized carbon material in the above solution, and ultrasonicate for 1h; then place it in an autoclave, Hydrothermal reaction at 220°C for 24 hours, naturally cooled to room temperature, washed with deionized water several times, dried to obtain a black powder, and ground to obtain a catalyst, which is recorded as CoS 2 @NCNH-800 2 .
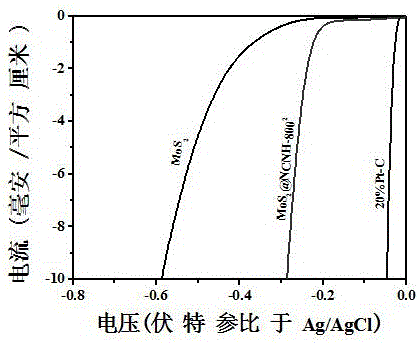
[0043] (4) CoS 2 @NCNH-800 2 Catalytic efficiency and effect: test method and condition are with embodiment 1. Test results: The c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Onset potential | aaaaa | aaaaa |
| Overpotential | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com