Novel process for synthesizing alkynol from acetylene and ketone compounds
A ketone compound and new process technology, which is applied in the field of synthesizing acetylenic alcohols from acetylene and ketone compounds, can solve the problems of not fully meeting the separation requirements, large compressor load, and large gas circulation volume, so as to reduce material circulation volume and reduce Compressor load, effect of increasing reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
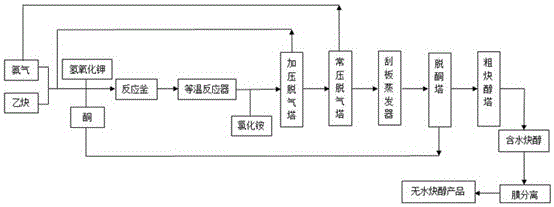
[0028] After mixing acetylene and ammonia in a molar ratio of 1:2 and compressing to 3MPa, add acetone and potassium hydroxide solution so that the wt% of potassium hydroxide in the reaction solution is 0.5%, and the molar ratio of acetylene to acetone is 1 : 1, then carry out the alkynylation reaction through the tank reactor and 2 isothermal tubular reactors successively, the reaction temperature is 40 ℃, the reaction pressure 3MPa, the reaction time is 120 minutes, then add ammonium chloride, ammonium chloride and hydrogen The molar ratio of potassium oxide was 1.5:1 to terminate the reaction.
[0029] After the reaction is terminated, the reaction neutralization liquid is sent to the pressurized degassing tower, separated at 3MPa, all acetylene and part of ammonia are extracted from the top of the tower, the molar ratio of the extracted acetylene to ammonia is 1:2, and part of the acetylene is The ammonia mixture is refluxed back to the pressurized degassing tower, and the...
Embodiment 2
[0036]Mix acetylene and ammonia in a molar ratio of 1:5 and compress to 5 MPa, then add acetone and potassium hydroxide solution, so that the mass concentration of potassium hydroxide in the reaction solution is 0.1%, and the molar ratio of acetylene to acetone is 5 : 1, then carry out the alkynylation reaction through the tank reactor and an isothermal tubular reactor successively, the reaction temperature is 5°C, the reaction pressure is 5MPa, and the reaction time is 10 minutes, then add ammonium chloride, ammonium chloride and hydrogen The molar ratio of potassium oxide was 1:1 to terminate the reaction.
[0037] After the reaction is terminated, the reaction neutralization liquid is sent to the pressurized degassing tower, separated at 5MPa, all acetylene and part of ammonia are extracted from the top of the tower, the molar ratio of the extracted acetylene to ammonia is 1:5, and part of the acetylene after condensation The ammonia mixture is refluxed back to the pressuri...
Embodiment 3
[0040] Mix acetylene and ammonia in a molar ratio of 1:4 and compress to 3MPa, then add butanone and potassium hydroxide solution so that the mass concentration of potassium hydroxide in the reaction solution is 0.3%, and the molar ratio of acetylene to butanone 4:1, and then through the tank reactor and two isothermal tubular reactors to carry out the alkynylation reaction, the reaction temperature is 40 ℃, the reaction pressure is 3MPa, the reaction time is 120 minutes, then add ammonium chloride, ammonium chloride The molar ratio with potassium hydroxide was 1.2:1 to terminate the reaction.
[0041] After the reaction is terminated, the reaction neutralization liquid is sent to the pressurized degassing tower and separated at 1.5 MPa. All the acetylene and part of the ammonia are extracted from the top of the tower. The molar ratio of the extracted acetylene to ammonia is 1:4. The mixed solution of acetylene and ammonia is refluxed back to the pressurized degassing tower, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com