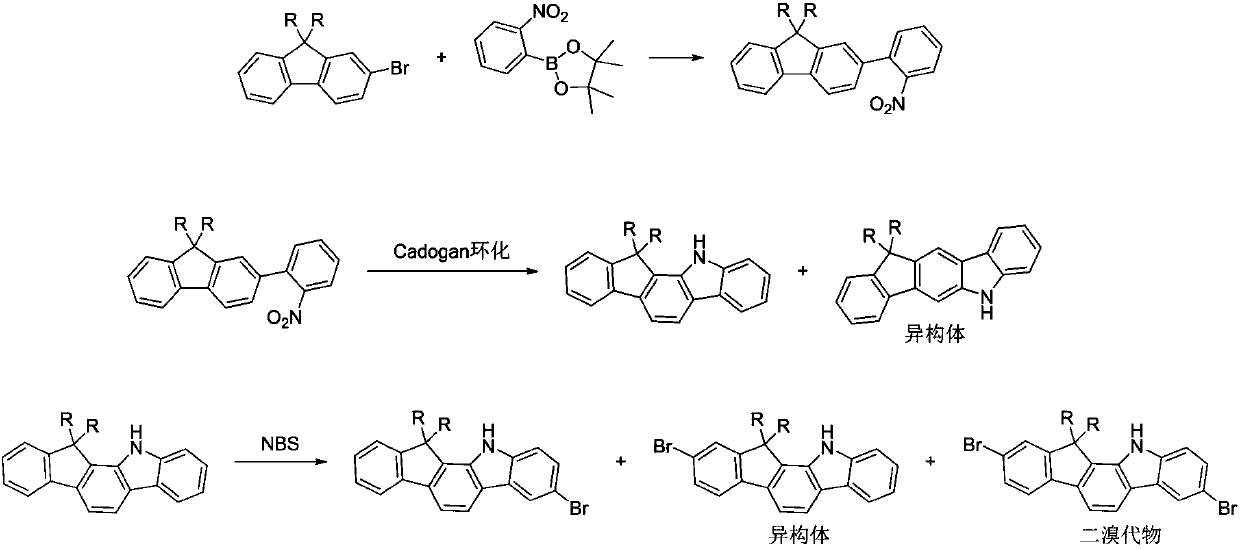
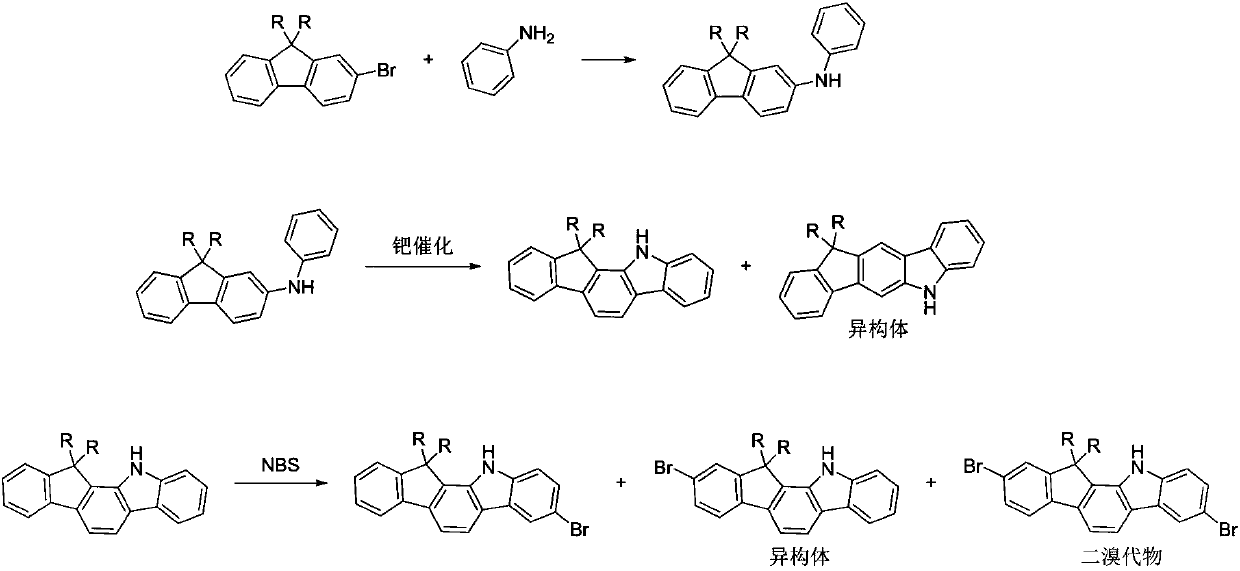
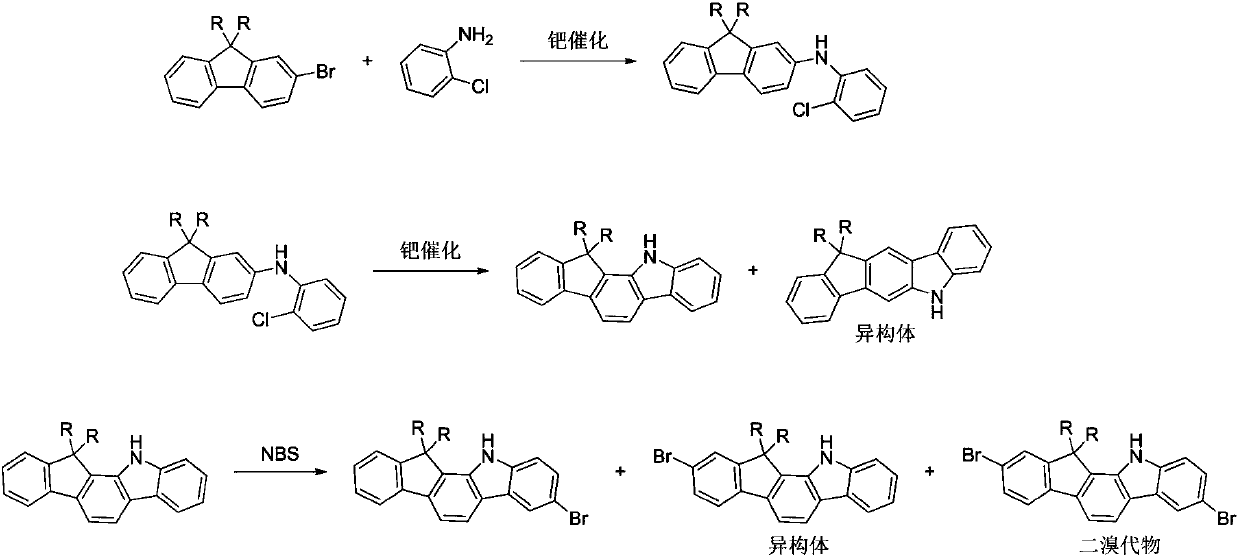
A kind of preparation method of fluorenyl benzindole derivative containing halogen
A technology for indole derivatives and fluorenyl benzene, which is applied in the field of organic synthesis, can solve the problems that the product is difficult to meet the organic optoelectronic materials, is difficult to meet the organic optoelectronic materials, affects the performance of finished materials, etc., and achieves low cost, broad market prospects, and low cost. reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of 2-chloro-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole
[0045] Preparation of 3-bromo-2-amino-9,9-dimethylfluorene: Dissolve 20.9g (100mmol) 2-amino-9,9-dimethylfluorene in 250mL chloroform solution, control internal temperature 0-5 ℃, add NBS 17.8g (100mmol) in batches, react the reaction mixture at 0-5℃ for 2.0hrs, add 100g 2.5% sodium bisulfite aqueous solution to the reaction system to quench the reaction, separate layers, wash the organic phase with 100mL water, anhydrous Drying over sodium sulfate and vacuum removal of the solvent gave 29.1 g of crude 3-bromo-2-amino-9,9-dimethylfluorene, which was then recrystallized using 8 g / g methanol to give 24.3 g of white solid powder with a yield of 84.37%.
[0046] Preparation of 3-(5-chloro-2-methoxyphenyl)-9,9-dimethyl-9H-fluorene-2-amine: 23.0g (80mmol) 3-bromo-2-amino-9, Add 9-dimethylfluorene, 14.9g (80mmol) 5-chloro-2-methoxyphenylboronic acid and 200mL toluene into a 1L three-necked fla...
Embodiment 2
[0051] Example 2: Preparation of 2-bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole
[0052] Preparation of 3-bromo-2-amino-9,9-dimethylfluorene: Dissolve 20.9g (100mmol) 2-amino-9,9-dimethylfluorene in 200mL dichloroethane solution, control the internal temperature -5-0°C, add NBS 16.9g (95mmol) in batches, react the reaction mixture at 0-5°C for 4.0hrs, add 100g of 2.5% sodium bisulfite aqueous solution to the reaction system to quench the reaction, separate layers, wash the organic matter with 100mL water phase, dried over anhydrous sodium sulfate, and vacuum desolvated to obtain 28.8 g of 3-bromo-2-amino-9,9-dimethylfluorene crude product, and then recrystallized using toluene ethanol mixed solvent to obtain 23.4 g of white solid powder, yield 81.19 %.
[0053] Preparation of 3-(5-bromo-2-methoxyphenyl)-9,9-dimethyl-9H-fluorene-2-amine: 23.0g (80mmol) 3-bromo-2-amino-9, Add 9-dimethylfluorene, 17.3g (75mmol) 5-bromo-2-methoxyphenylboronic acid and 250mL toluene into ...
Embodiment 3
[0058] Example 3: Preparation of 2-bromo-7,7-diphenyl-5,7-dihydroindeno[2,1-b]carbazole
[0059] Preparation of 3-bromo-2-amino-9,9-diphenylfluorene: Dissolve 33.3g (100mmol) 2-amino-9,9-diphenylfluorene in 300mL dichloromethane solution, control the internal temperature for 10 -15°C, add NBS 18.2g (102mmol) in batches, react the reaction mixture at 10-15°C for 8.0hrs, add 100g to the reaction system, quench the reaction with 2.5% aqueous sodium bisulfite solution, separate layers, wash the organic phase with 100mL water, Drying over anhydrous sodium sulfate, vacuum removal of the solvent yielded 41.3 g of crude 3-bromo-2-amino-9,9-diphenylfluorene, and then recrystallized using toluene ethanol mixed solvent to obtain 33.6 g of white solid powder with a yield of 81.55%.
[0060] Preparation of 3-(5-bromo-2-methoxyphenyl)-9,9-diphenyl-9H-fluorene-2-amine: 33.0g (80mmol) 3-bromo-2-amino-9, Add 9-diphenylfluorene, 17.3g (75mmol) 5-bromo-2-methoxyphenylboronic acid and 250mL tolu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com