Enhanced fluorescent probe and preparation method and application thereof
A fluorescent probe and enhanced technology, applied in the field of enhanced fluorescent probes and their preparation, can solve the problems of poor water solubility, difficult preservation of azoreductase, and the need to add a large amount of organic solvent for testing, and achieves high sensitivity, high sensitivity, and high sensitivity. High accuracy and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
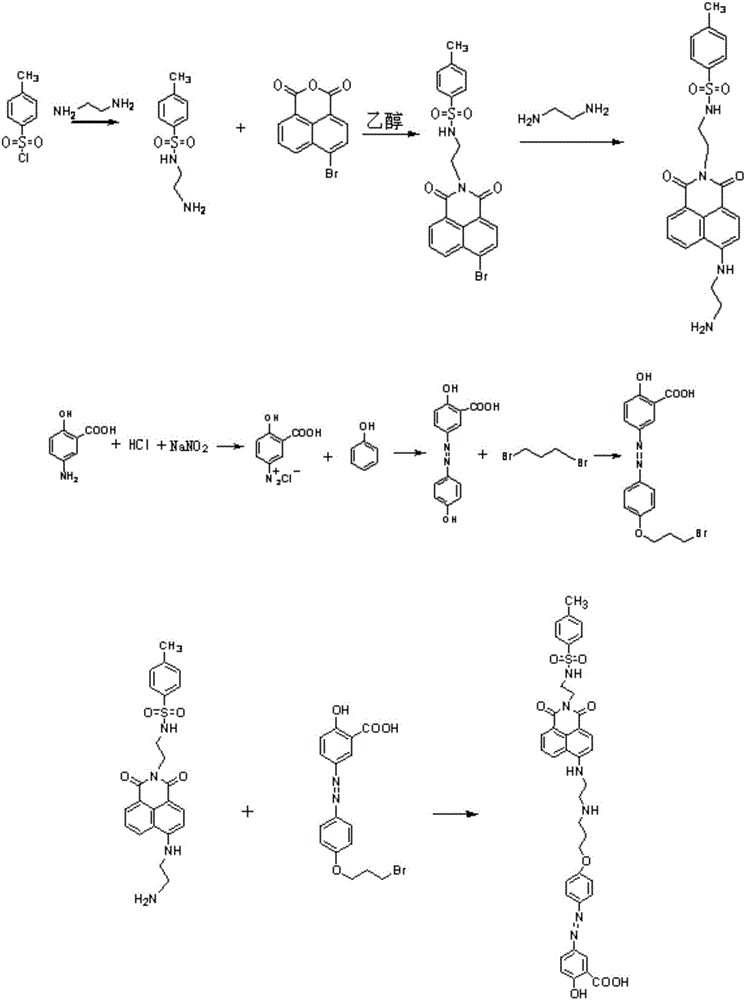
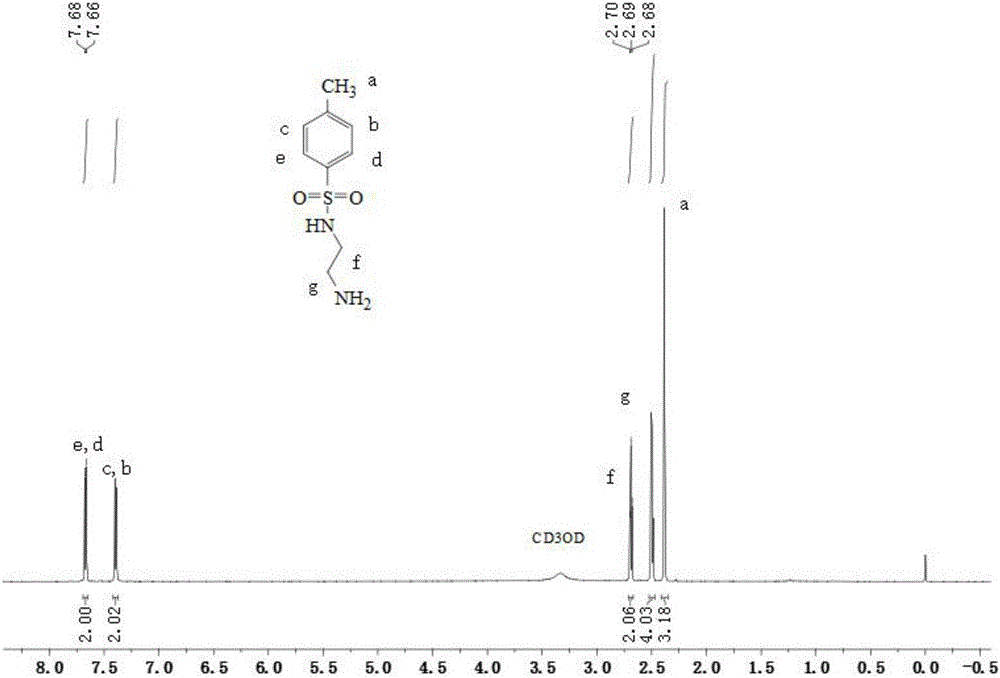
[0043] (1) 3g of 4-methyl-benzenesulfonyl chloride (15.8mmol) was dissolved in 8mL of dichloromethane, and was added dropwise to 9.48g of ethylenediamine (158mmol) under conditions of ice bath and nitrogen protection, and the resulting mixture The solution was stirred overnight at room temperature. After the reaction was completed, the reaction mixture was extracted three times with dichloromethane and potassium carbonate aqueous solution, and the organic phase was collected, dried over magnesium sulfate, and filtered; the resulting solid was purified by silica gel chromatography (eluent used was dichloromethane / methanol , V / V=8:1), the organic solvent was removed by rotary evaporation to obtain solid N-(2-amino-ethyl)-4-methyl-benzenesulfonamide. It was characterized by H NMR spectroscopy: 1 H NMR (400MHz, CD 3 OD-d 4 ,δppm):7.65-7.69ppm(d,2H), 7.37-7.42ppm(d,2H), 2.66-2.71ppm(t,2H), 2.47-2.52ppm(m,4H), 2.35-2.41ppm(s ,3H). Among them, 2.36ppm is the characteristic peak ...
Embodiment 2
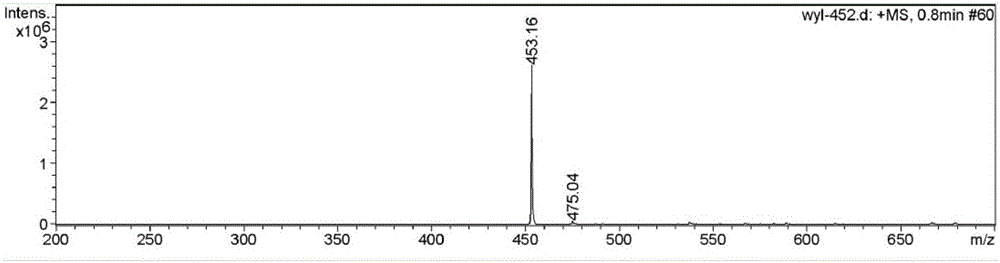
[0048] (1) Take 3.6g of 4-methyl-benzenesulfonyl chloride (18.94mmol) and dissolve it in 18.94mL of dichloromethane, and add it dropwise to 11.5g of ethylenediamine (191.3mmol) under the conditions of ice bath and nitrogen protection , and the resulting mixed solution was stirred overnight at room temperature. After the reaction was completed, the reaction mixture was extracted three times with dichloromethane and potassium carbonate aqueous solution, and the organic phase was collected, dried over magnesium sulfate, and filtered; the resulting solid was purified by silica gel chromatography (eluent used was dichloromethane / methanol, V / V=8:1), the organic solvent was removed by rotary evaporation to obtain solid N-(2-amino-ethyl)-4-methyl-benzenesulfonamide. Take the N-(2-amino-ethyl)-4-methyl-benzenesulfonamide solid (516mg, 2.4mmol) obtained above and dissolve it in 4-bromo-1,8-naphthalic anhydride (664mg, 2.4mmol) In 30mL of ethanol, stir to dissolve it completely; the re...
Embodiment 3
[0054] (1) 4.7g of 4-methyl-benzenesulfonyl chloride (24.5mmol) was dissolved in 49mL of dichloromethane, and was added dropwise to 14.96g of ethylenediamine (249.9mmol) under conditions of ice bath and nitrogen protection, The resulting mixed solution was stirred overnight at room temperature. After the reaction was completed, the reaction mixture was extracted three times with dichloromethane and potassium carbonate aqueous solution, and the organic phase was collected, dried over magnesium sulfate, and filtered; the resulting solid was purified by silica gel chromatography (eluent used was dichloromethane / methanol, V / V=8:1), the organic solvent was removed by rotary evaporation to obtain solid N-(2-amino-ethyl)-4-methyl-benzenesulfonamide. Take N-(2-amino-ethyl)-4-methyl-benzenesulfonamide (671mg, 3.12mmol) and 4-bromo-1,8-naphthalic anhydride (863mg, 3.12mmol) obtained above were dissolved in In 46.8mL of ethanol, stir to dissolve it completely; the resulting solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com