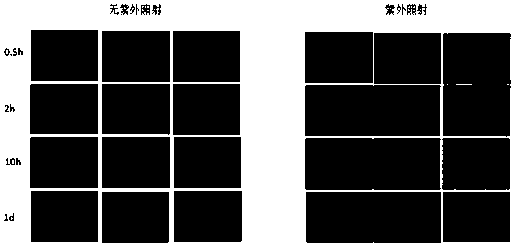
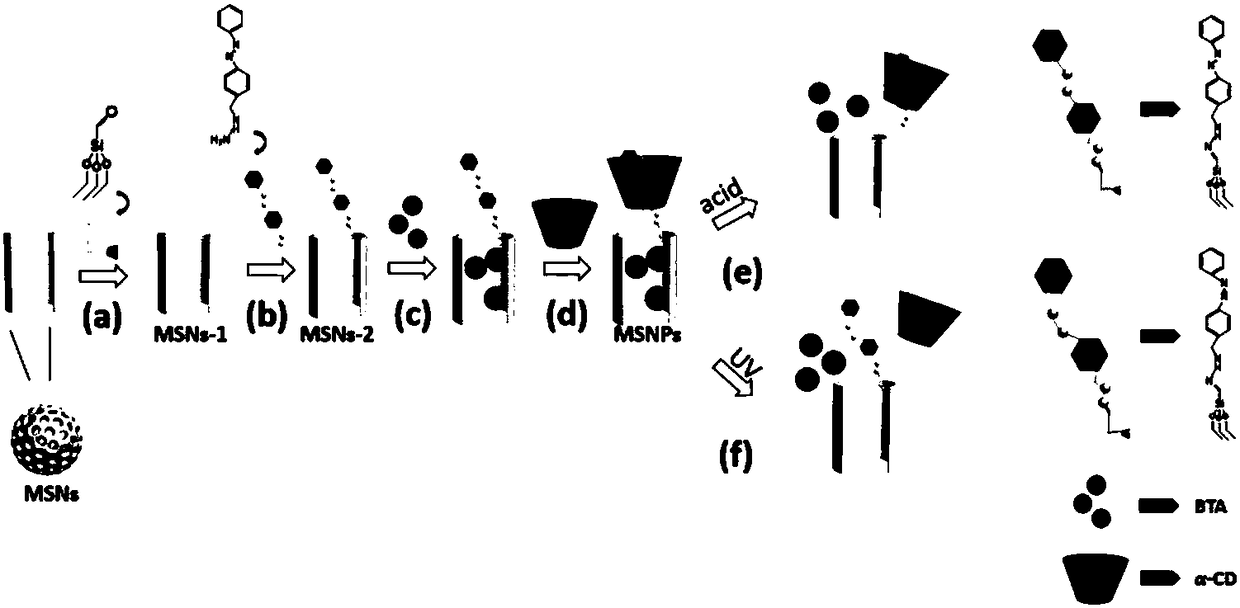
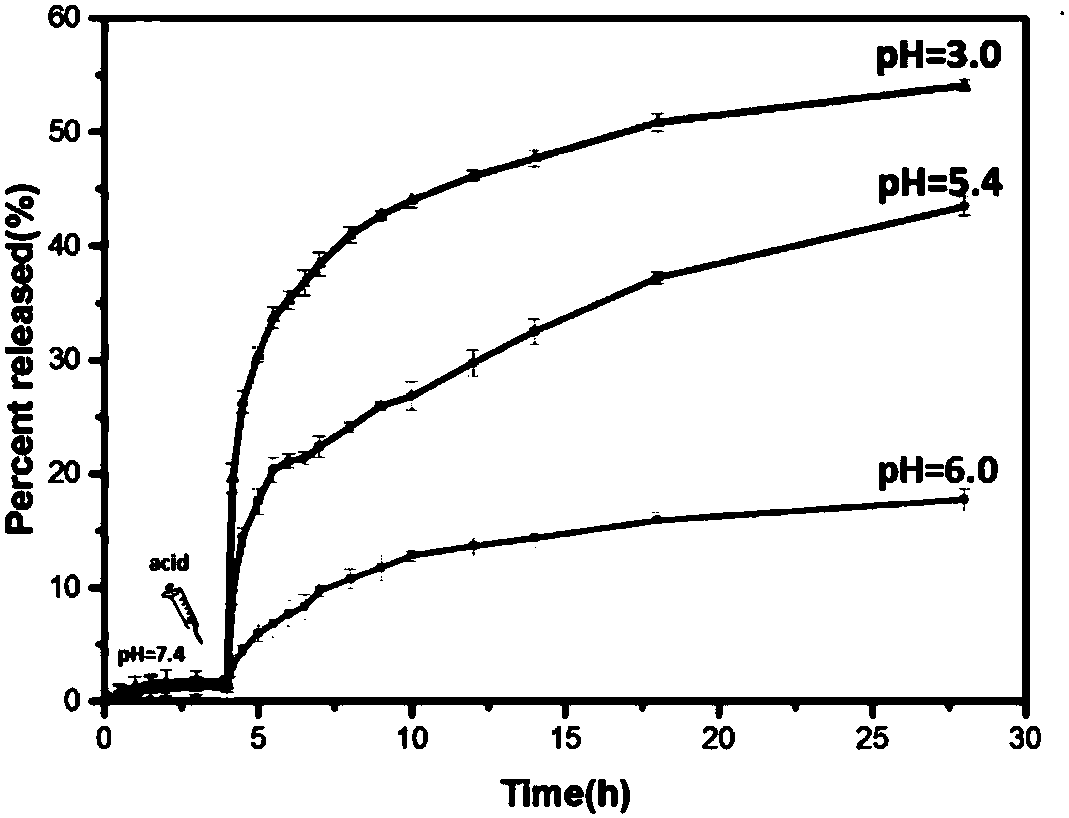
A kind of ph and light dual response targeting drug loading system and its preparation method
A dual-response, targeted technology, applied in the field of biochemistry, can solve the problems of low drug release and release rate, and achieve the effect of easy adjustment, good application prospect and favorable reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Synthesis of aldehyde triethoxysilane and hydrazinomethylazobenzene
[0035] Stir 2mL of vinyltriethoxysilane in 15mL of dichloromethane, keep the temperature at -90°C with liquid nitrogen and ethanol, pass ozone into the solution until the solution turns light blue, and blow off excess ozone with nitrogen, Add 2.488g of triphenylphosphine, leave the mixture overnight, dry with anhydrous sodium sulfate, and rotary evaporate to obtain aldehyde triethoxysilane.
[0036]Dissolve 4.66mL of hydrazine hydrate (0.1mol) in 200mL of chloroform, otherwise known as 4.4g Diboc (0.02mol) in 20mL of chloroform, and add hydrazine hydrate three times (5mL each time) dropwise under ice bath conditions. Chloromethane solution (dropped within 24h), and then reacted at room temperature for 24h. After concentrating by rotary evaporation, 100 mL of ethyl acetate was added to dissolve the product, and extracted three times with half-saturated brine, 50 mL each time. The product was dried...
Embodiment 2
[0047] 1. Synthesis of aldehyde triethoxysilane and hydrazinomethyl azobenzene
[0048] Stir 2mL of vinylmethoxysilane in 15mL of dichloromethane, keep the temperature at -70°C with liquid nitrogen and ethanol, pass ozone into the solution until the solution turns light blue, blow away excess ozone with nitrogen, add 2. 488g of triphenylphosphine, the mixture was left overnight, dried with anhydrous sodium sulfate, and rotary evaporated to obtain aldehyde methoxysilane.
[0049] Dissolve 4.66mL of hydrazine hydrate (0.1mol) in 200mL of chloroform, and dissolve 2.2g of Diboc (0.01mol) in 20mL of chloroform. Under ice-bath conditions, add hydrazine hydrate three times (5mL each time) dropwise. Chloromethane solution (dropped within 24h), and then reacted at room temperature for 24h. After concentrating by rotary evaporation, 100 mL of ethyl acetate was added to dissolve the product, and extracted three times with half-saturated brine, 50 mL each time. The product was dried ove...
Embodiment 3
[0060] 1. Synthesis of aldehyde triethoxysilane and hydrazinomethyl azobenzene
[0061] Stir 2mL of vinyltriethoxysilane in 15mL of dichloromethane, keep the temperature at -78°C with liquid nitrogen and ethanol, pass ozone into the solution until the solution turns light blue, and blow away excess ozone with nitrogen, Add 2.488g of triphenylphosphine, leave the mixture overnight, dry with anhydrous sodium sulfate, and rotary evaporate to obtain aldehyde triethoxysilane.
[0062] Dissolve 4.66mL of hydrazine hydrate (0.1mol) in 200mL of chloroform, otherwise known as 4.4g Diboc (0.02mol) in 20mL of chloroform, and add hydrazine hydrate three times (5mL each time) dropwise under ice bath conditions. Chloromethane solution (dropped within 24h), and then reacted at room temperature for 24h. After concentrating by rotary evaporation, 100 mL of ethyl acetate was added to dissolve the product, and extracted three times with half-saturated brine, 50 mL each time. The product was dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com