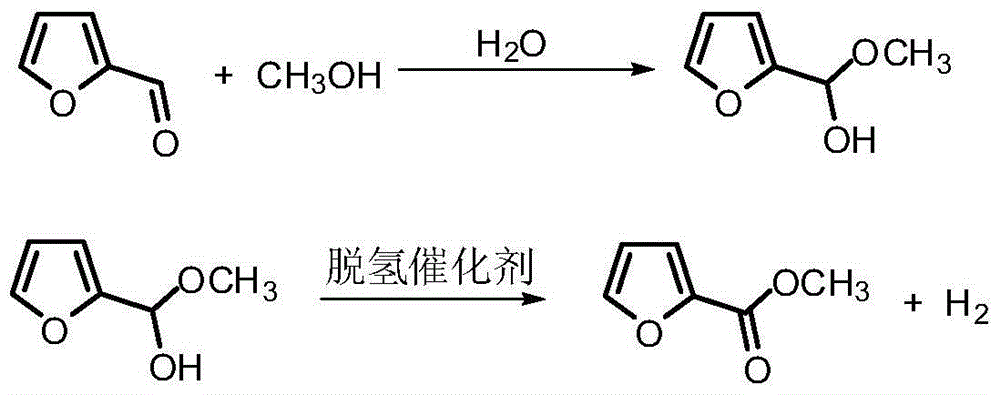
Method for preparing methyl 5-hydroxypentanoate
A technology of methyl hydroxyvalerate and methyl furoate, which is applied in the field of preparation of methyl 5-hydroxyvalerate, and can solve the problems of unsatisfactory production of methyl 5-hydroxyvalerate, cumbersome catalyst preparation, poor reaction selectivity, etc. problems, to avoid polymerization side reactions, inhibit the formation of by-products, and achieve the effect of easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0039] Embodiment 1~11: preparation of dehydrogenation catalyst
[0040] Prepare ruthenium chloride into a 10wt% aqueous solution, add carrier powder according to the ratio in Table 1, stir and mix evenly, impregnate at room temperature for 12 hours, dry at 120°C for 12 hours, and roast at 500°C for 4 hours; if the carrier is activated carbon, no roasting is required. Catalyst raw powders with different active component contents are prepared, and a shaped dehydrogenation catalyst precursor is obtained through tablet molding.
[0041] Table 1 Preparation of dehydrogenation catalyst
[0042] Catalyst serial number carrier Ru content wt% 1# activated carbon 5 2# SiO 2 4 3# al 2 o 3 5 4# TiO 2 3 5# MgO 2 6# SiO 2 -Al 2 o 3 (mass ratio 1:1) 4 7# SiO 2 -TiO 2 (mass ratio 4:1) 4 8# SiO 2 -MgO (mass ratio 1:2) 5 9# TiO 2 -Al 2 o 3 (mass ratio 2:3) 3 10# MgO-Al 2 o 3 (mass ratio 3:2)...
Embodiment 12~22
[0043] Embodiment 12~22: hydrogenolysis catalyst preparation
[0044] Prepared by a step-by-step equal-volume impregnation method, adding a solution of 10 wt% soluble salt of the active component into the carrier according to a certain metering ratio, stirring evenly, impregnating at room temperature for 12 hours, and then drying in an oven at 120°C for 12 hours; Calcining in air at 500° C. for 3 hours to obtain the original catalyst powder, and forming a shaped hydrogenolysis catalyst precursor by tablet molding.
[0045] Table 2 Hydrogenolysis Catalyst Preparation
[0046]
[0047]
Embodiment 23
[0048] Example 23: Catalyst Evaluation
[0049] The 1# reactor is a stainless steel tube with an outer diameter of 40mm, an inner diameter of 20mm, and a length of 1000mm. Load 50g of dehydrogenation catalyst precursor into 1# reactor, and reduce the catalyst precursor in situ before the reaction, the reduction temperature is 250°C, H 2 The pressure is 0.2MPa, H 2 The flow rate is 1.5L / min, and the reduction takes 4 hours.
[0050] The 2# reactor is a stainless steel tube with an outer diameter of 40mm, an inner diameter of 20mm, and a length of 1000mm. Fill 50g of the hydrogenolysis catalyst precursor into the 2# reactor, and reduce the catalyst precursor in situ before the reaction, the reduction temperature is 300°C, H 2 The pressure is 0.5MPa, H 2 The flow rate is 1.5L / min, and the reduction takes 4 hours.
[0051] Furfural, methanol, and water enter the 1# reactor, and take samples and analyze at the outlet of the 1# reactor; the reaction liquid and hydrogen of the 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com