Synthesis and application of photosensitive medicament taking folic acid as targeted group
A photosensitive and targeted technology, which can be used in drug combinations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. It can solve problems such as hindering application, limited ability of Pyro tumor localization, and tissue damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
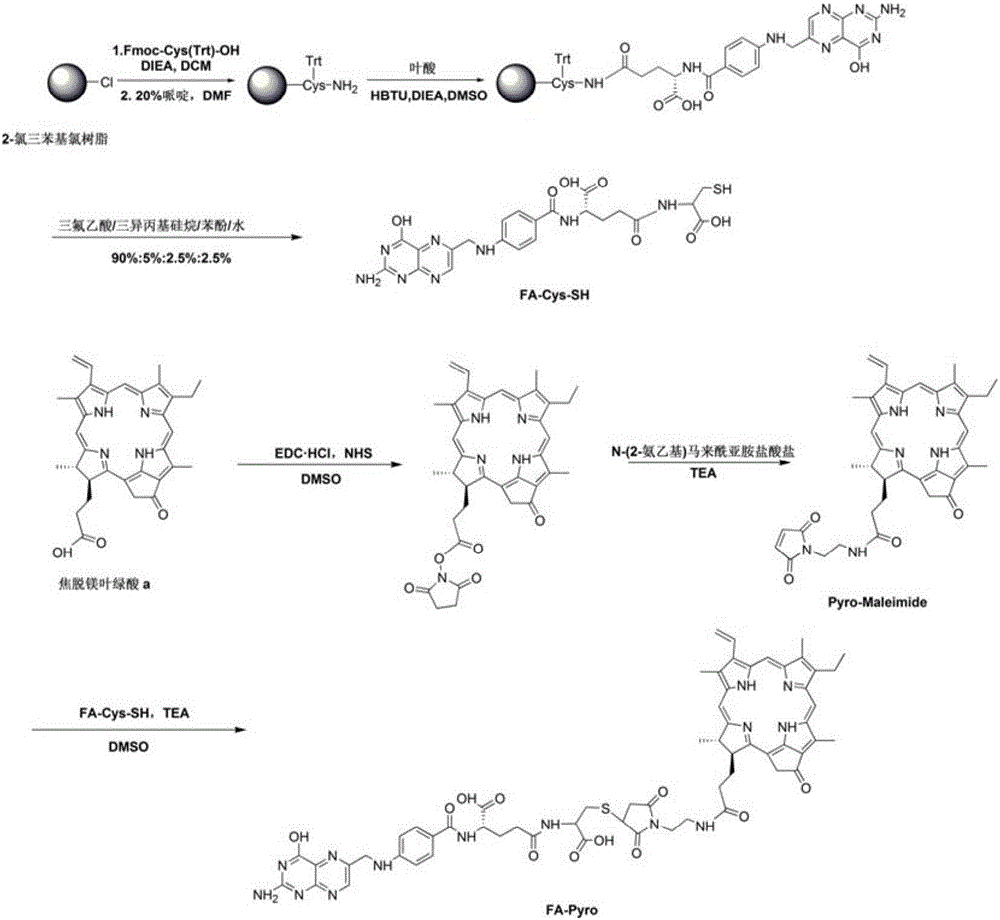
[0035] The compound that is called for short Pyro-Maleimide and the compound that is called FA-Pyro for short (attachment figure 1 ).
[0036] The synthesis process integrates the solid-phase synthesis method and the liquid-phase synthesis method, and facilitates large-scale preparation on the basis of simplifying the post-treatment process. Among them, the synthesis of FA-Pyro adopts the classic reaction method of thiol and maleimide, which greatly improves the yield of the compound.
[0037] 1. Synthesis of a compound called FA-Cys-SH for short
[0038] FA-Cys-SH is synthesized by Fmoc-based solid phase synthesis. Weigh 1g of 2-chlororitylchloride resin (1g, 0.5mmol, 1.0eq) into a solid-phase synthesizer, add 10ml of dichloromethane (DCM) to swell for 20min. Fmoc-Cys(Trt)-OH (351mg, 0.6mmol, 1.2eq) and N,N-diisopropylethylamine (DIEA, 198μl, 1.2mmol, 2.4eq) were dissolved in 8ml DCM and added to the solid-phase synthesis Reactor at room temperature for 4h. Configure a b...
Embodiment 2
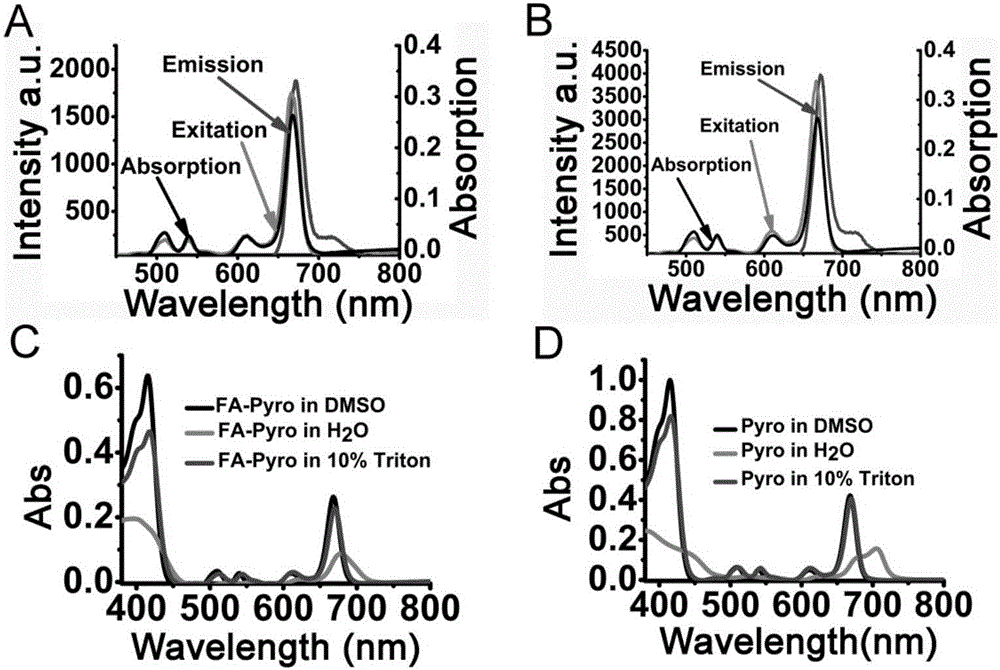
[0047] Spectral properties of FA-Pyro and Pyro (attached figure 2 ).
[0048] (1) UV-Vis spectrum
[0049] The absorption spectra of FA-Pyro and Pyro were scanned with USA Cary 5000 in the wavelength range 300–850. The concentrations of FA-Pyro and Pyro were 10 μM, respectively.
[0050] (2) Fluorescence excitation and emission spectra
[0051] The fluorescence emission spectrum uses 665nm as the excitation light, and the recording spectrum ranges from 600-900nm. The fluorescence excitation spectrum uses 675 as the emission light, and the recording excitation light range is 450-800nm. The concentration of FA-Pyro and Pyro was recorded as 5 μM. The photomultiplier tube (PMT) voltage is 700V, and the scanning speed is 2400nm / min.
[0052] Experimental results and conclusions:
[0053] The spectral properties of FA-Pyro and Pyro are the absorption spectrum and fluorescence spectrum properties such as figure 2 As shown in A and 2B: in DMSO, the spectral properties of FA-...
Embodiment 3
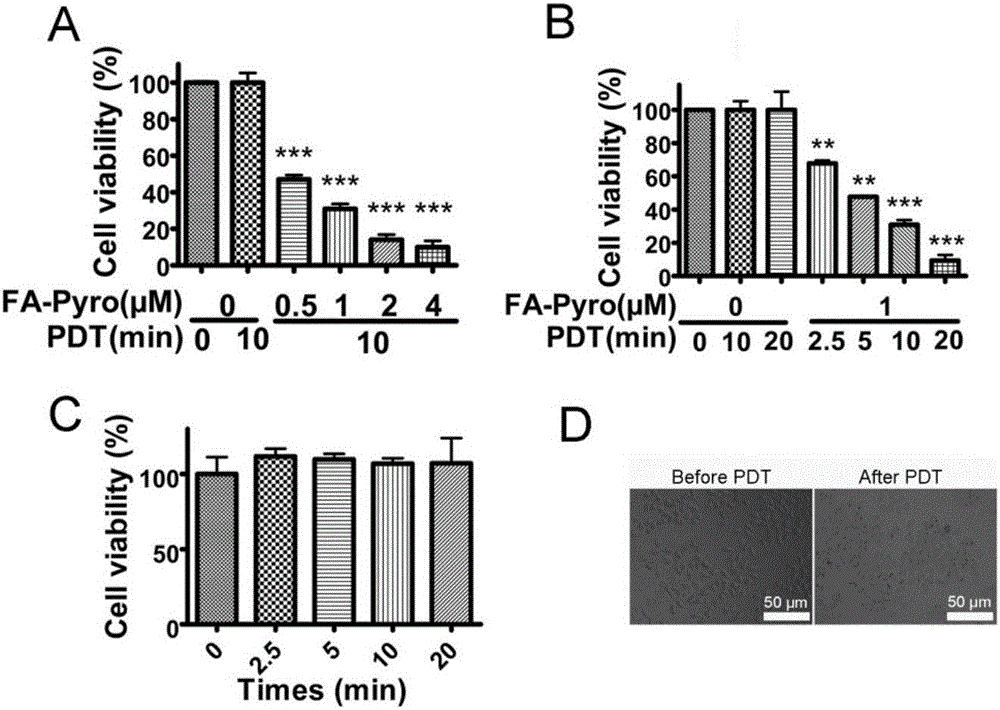
[0055] Compound FA-Pyro's ability to kill tumor cells (attached image 3 ).
[0056] 1) Culture of KB (human oral epidermal carcinoma cell) and A549 (human lung bronchial carcinoma cell) cell lines
[0057] The cells were taken out of liquid nitrogen, placed in a 37°C water bath to thaw quickly, and then centrifuged at 1000rpm / min for 5min. Replace the supernatant, add preheated complete medium, and place in a 5% CO2, 37°C incubator to culture overnight. The medium was changed the next day. Continue to culture until the cells cover the bottom of the dish. Subculture 2-3 times.
[0058] 2) drug and cell incubation
[0059] Take the cells in the logarithmic phase, with 5×10 3 Add 100 μL of cell suspension to a 96-well plate at a cell density of 1 cell / well, and incubate for 24 hours in a 37°C incubator. After aspirating the medium, add fresh medium containing different FA-Pyro concentrations to continue culturing for 6 hours, use 660nm, 40-mW / cm 2 Give different light do...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com