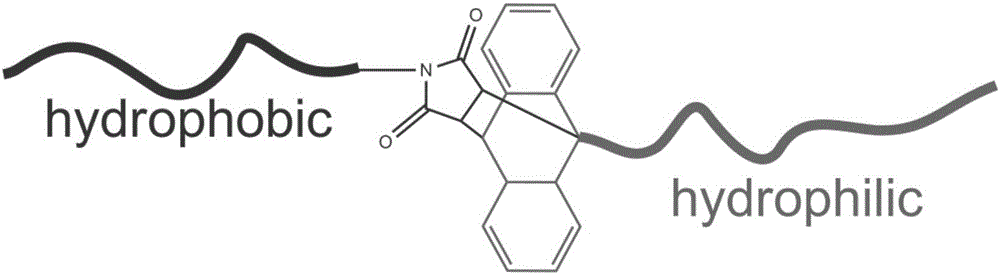
Preparation method of environment-friendly macromolecular emulsifier
A macromolecular emulsifier, an environment-friendly technology, applied in the field of preparation of an environment-friendly macromolecular emulsifier, can solve the problems of water pollution, consumption of dissolved oxygen, difficulty in the process of removing the emulsifier, etc., and achieves mild reaction conditions and operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of a kind of environment-friendly macromolecular emulsifier
[0027] Add 8 parts of tert-butyl methacrylate, 1 part of ligand tris(2-dimethylaminoethyl)amine, 1 part of cuprous bromide, and 0.05 part of functional anthracene initiator in the reaction tube equipped with magneton, 8 parts of dimethyl sulfoxide, after three freeze-thaw cycles to remove oxygen and water in the system, reacted at room temperature under the protection of an inert gas for 7 hours, after the reaction was completed and purified, the end-group anthracene functionalized poly-tert-butyl methacrylate was obtained. segment;
[0028] Add 8 parts of butyl methacrylate, 1 part of ligand pentamethyldiethylenetriamine, 0.05 part of functionalized maleic anhydride initiator, 1 part of copper bromide, 8 parts of amide, after three freeze-thaw cycles to remove oxygen and water in the system, add the treated 0.5cm copper wire, and react at room temperature under the protection o...
Embodiment 2
[0031] Embodiment 2: the preparation of a kind of environment-friendly macromolecular emulsifier
[0032] Add 8 parts of tert-butyl acrylate, 2 parts of ligand pentamethyldiethylenetriamine, 1 part of cuprous bromide, 0.05 parts of functional anthracene initiator, 8 parts of dimethyl sulfoxide into the reaction tube equipped with magnetons. After three freeze-thaw cycles to remove oxygen and water in the system, react at room temperature under the protection of an inert gas for 5 hours, after the reaction is completed and purified, a long chain segment of poly(tert-butyl acrylate) functionalized with anthracene is obtained;
[0033] Add 8 parts of butyl acrylate, 2 parts of ligand pentamethyldiethylenetriamine, 0.05 part of functionalized maleic anhydride initiator, 1 part of copper bromide, 8 parts of dimethylformamide in the reaction tube equipped with magneton After three freeze-thaw cycles to remove oxygen and water in the system, add the treated 0.5cm copper wire, and rea...
Embodiment 3
[0036] Embodiment 3: the preparation of a kind of environment-friendly macromolecular emulsifier
[0037] Add 8 parts of tert-butyl acrylate, 2 parts of ligand 4,4'-dinonyl-2,2'-bipyridine, 1 part of cuprous bromide, functional anthracene initiator into the reaction tube equipped with magneton 0.1 parts, 8 parts of dimethylformamide, after three refrigeration cycles to remove oxygen and water in the system, react at room temperature under the protection of an inert gas for 10 hours, after the reaction is completed and purified, the terminal anthracene-functionalized poly(tert-butyl acrylate) is obtained. segment;
[0038] Add 8 parts of styrene, 2 parts of ligand tris(2-dimethylaminoethyl)amine, 0.1 part of functionalized maleic anhydride initiator, 1 part of copper bromide, and 8 parts of toluene in the reaction tube equipped with magneton , after removing oxygen and water in the system through three freezing cycles, add a treated 1cm copper wire, and react at room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com