Preparation method of Ni-based catalyst with adjustable selectivity/non-selectivity
A non-selective, catalytic technology, applied in the new field of catalysis, can solve the problems such as the inability to directly realize the selective and adjustable catalytic process, and achieve the effect of large-scale industrial production, simple technical principle, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
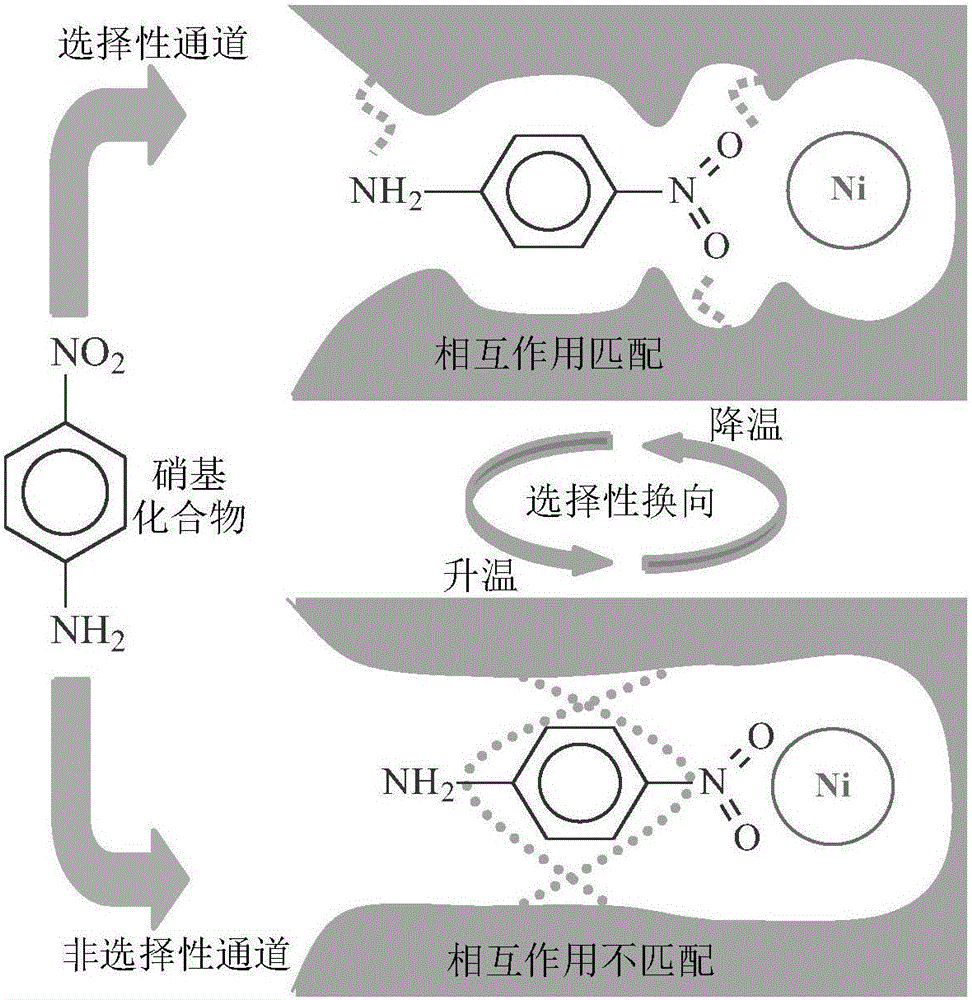
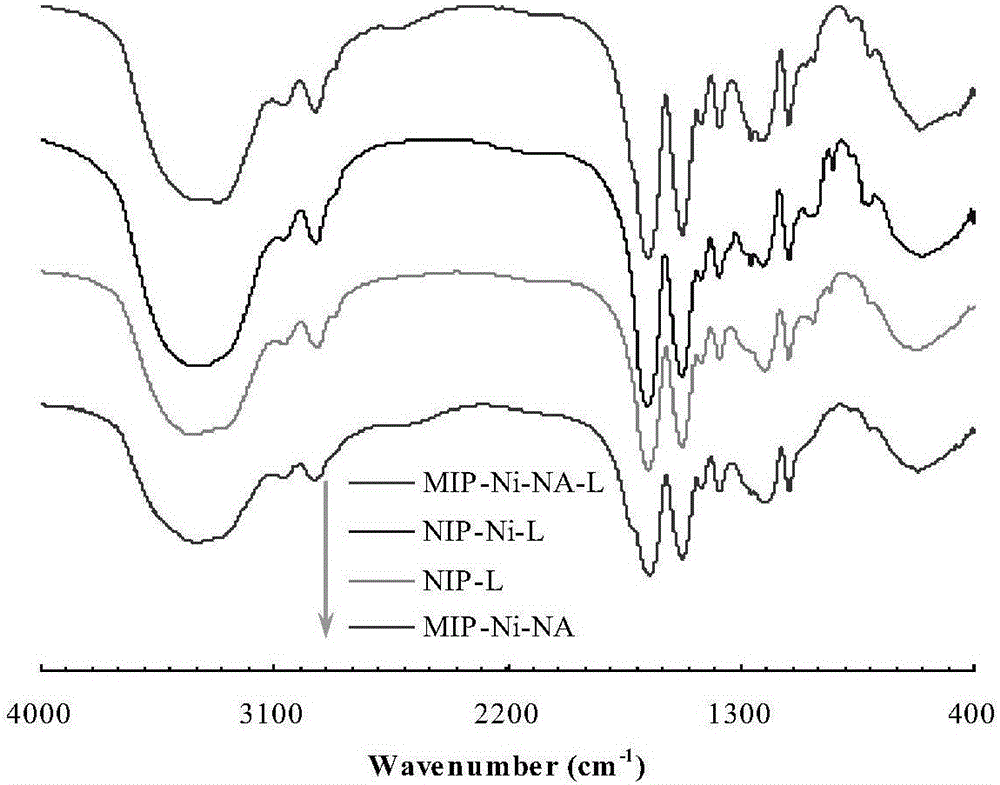
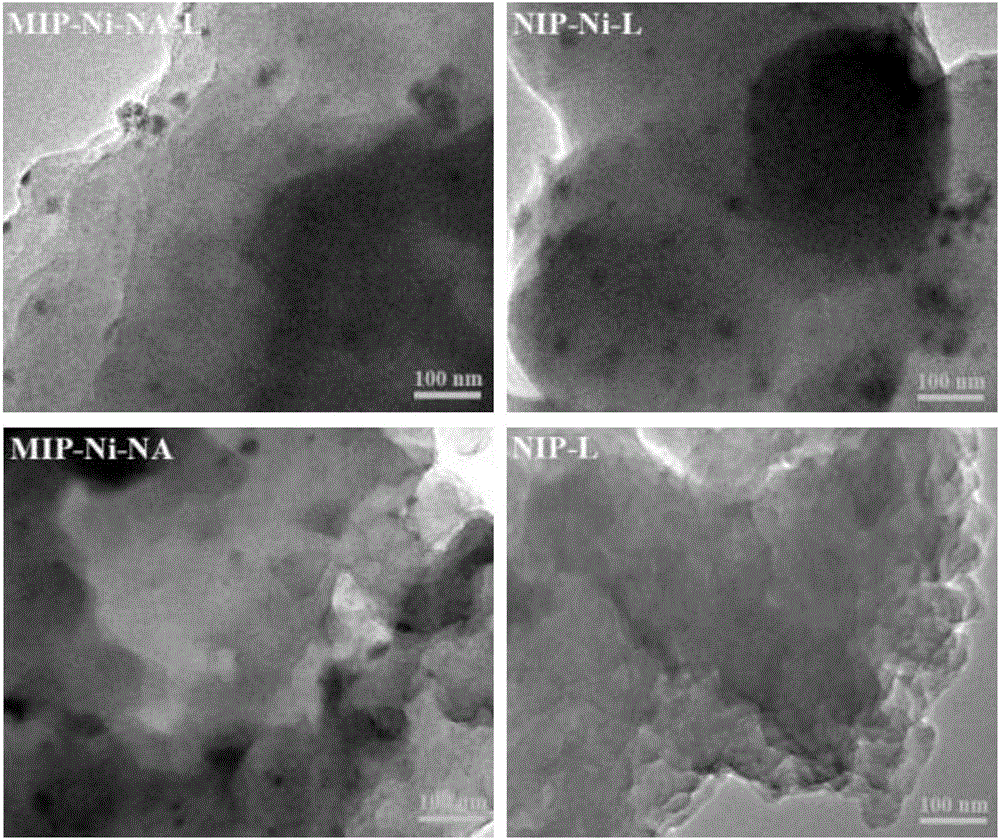
[0034] Dissolve the substrate p-nitroaniline (0.5mmol) and the active component precursor nickel nitrate (0.25mmol) in dimethyl sulfoxide (10mL), after ultrasonic dispersion and compounding for 25 minutes, add the functional monomer 7-octyl Acrylic acid (1.0mmol), cross-linking agent N, N methylenebisacrylamide (3.5mmol) and initiator azobisisobutyronitrile (0.1g), then nitrogen deoxygenation into the solution for 8 minutes, sealed and placed Polymerization was initiated by irradiation under ultraviolet light (24 hours) to form a catalyst precursor. The Ni ions in the catalyst precursor were reduced by an excessive amount of sodium borohydride (2.5mmol), and the obtained product was washed with water repeatedly after the imprinted p-nitroaniline was eluted with a mixed solution of ethanol (90vol-%)-acetic acid (10vol-%), and vacuum Drying to obtain a Ni-based catalyst with adjustable selectivity (for its technical principle, see figure 1 ; labeled "MIP-Ni-NA-L", where MIP is ...
Embodiment 2
[0041] Operated with Example 1, the long-chain functional monomer was changed from 7-octenoic acid to 6-heptenoic acid (1.0mmol), and the others were completely operated with Example 1 to obtain a Ni base with a phase transition point of about 39°C. Catalyst MIP-Ni-NA-L. Under the same catalytic test conditions as in Example 1, select 35 and 45°C higher and lower than the phase transition point for comparative measurement, the results are shown in Figure 8 . At 35°C, the prepared MIP-Ni-NA-L catalyst showed selective catalytic effect on the specific substrate p-nitroaniline; in contrast, at 45°C, MIP-Ni-NA -L shows no selectivity for the specific substrate p-nitroaniline and its analog m-nitroaniline. The prepared MIP-Ni-NA-L catalyst also exhibits the characteristics of "selective / non-selective" tunable catalysis.
Embodiment 3
[0043] The operation was the same as in Example 1, except that the specific substrate was changed to o-nitroaniline, and the other preparations were exactly the same as in Example 1 to obtain a Ni-based catalyst MIP-Ni-NA-L with a phase transition point of about 37°C. Under the same catalytic test conditions as in Example 1, the catalytic test substrate is changed to the specific substrate o-nitroaniline and analog m-nitroaniline, and the temperature is selected to be higher than and lower than the phase transition point at 25 and 40°C. For comparison, the results can be seen in Figure 9 . At 25 °C, the prepared MIP-Ni-NA-L catalyst exhibited selective catalytic effects on the specific substrate o-nitroaniline; in contrast, at 40 °C, MIP-Ni-NA -L shows no selectivity for the specific substrate o-nitroaniline and its analog m-nitroaniline. The prepared MIP-Ni-NA-L catalyst also exhibits the characteristics of "selective / non-selective" tunable catalysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com