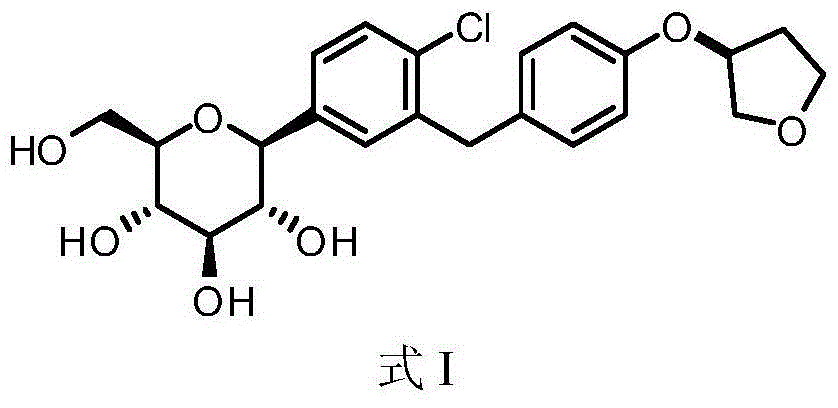
Empagliflozin monocrystalline and preparation method and purpose thereof
A technology of empagliflozin and single crystal, which is applied in the field of empagliflozin single crystal and its preparation, can solve the problems that the preparation method of empagliflozin single crystal needs to be improved, and achieve good shape, good reproducibility, and guaranteed The effect of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 The synthesis of empagliflozin crude product
[0041] According to the following steps to synthesize the crude product of Empagliflozin, the specific synthesis method is as follows:
[0042] Step 1 synthesizes 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone, the specific method is as follows:
[0043] (1) Add 800 grams of tetrahydrofuran, 100 grams of gluconolactone, and 454 grams of N-methylmorpholine to the reaction flask at room temperature. Under nitrogen protection, start to add 366 grams of trimethylchlorosilane dropwise. After the reaction is complete, Add ice water to quench the reaction.
[0044] (2) Add 2.6 kg of purified water and 500 g of n-heptane to the reaction solution in step (1), stir, leave to stand and separate the layers, and separate the organic layer.
[0045] (3) Wash the organic layer with 1.5 kg of 5% sodium dihydrogen phosphate aqueous solution, 1.5 kg of purified water and 1.5 kg of sodium chloride solution respectively.
[00...
Embodiment 2
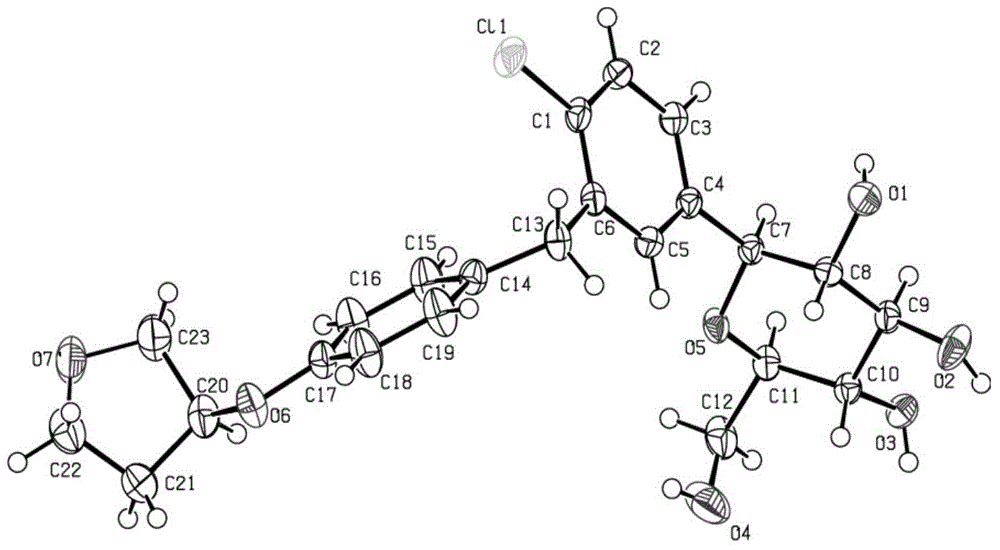
[0061] Embodiment 2 Preparation of Empagliflozin Single Crystal
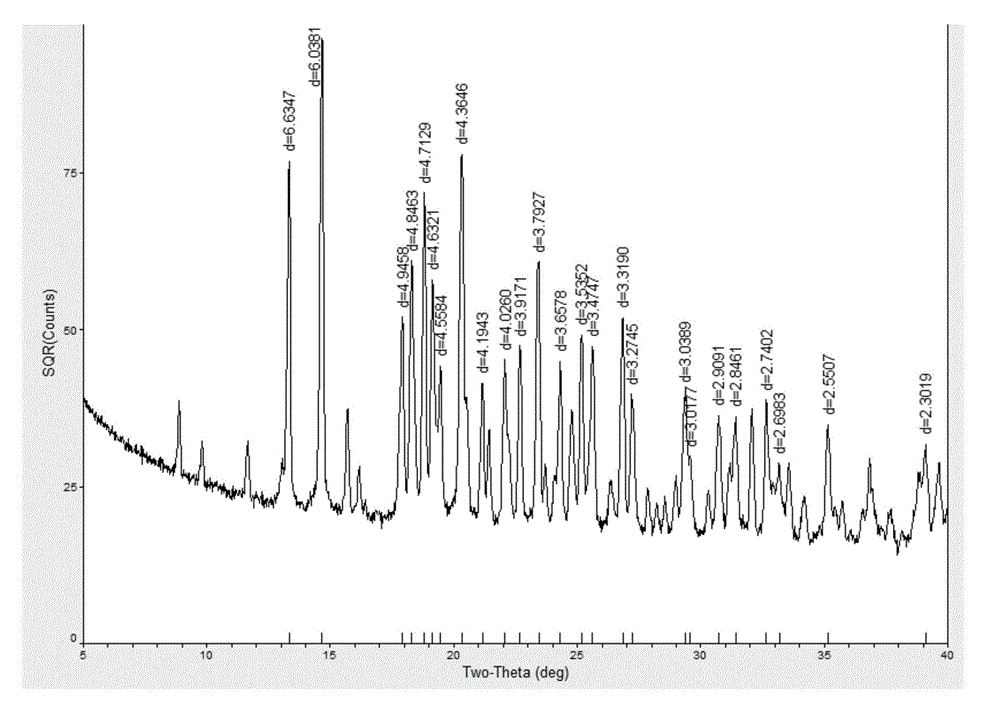
[0062] Weigh 0.20 g of the crude product of Empagliflozin prepared in Example 1, add 20 ml of crystallization solvent methanol, and wait until the product is completely dissolved, use the solvent slow evaporation method, set the temperature at 10-20°C, and cultivate for 20 days, colorless flakes Crystals are precipitated, and the crystals are ggliflozin single crystals, the yield of the ggliflozin single crystals is 80%, and the purity is 99.6%. Using X-ray powder diffractometer to detect, Empagliflozin single crystal X-ray powder diffraction pattern is as follows figure 1 shown.
Embodiment 3
[0063] Example 3 Preparation of Empagliflozin Single Crystal
[0064] Weigh 0.20 g of the crude product of Empagliflozin prepared in Example 1, add 20 ml of crystallization solvent ethanol, wait until the product is completely dissolved, use the solvent slow evaporation method, set the temperature at 10-20 °C, and cultivate for 25 days, there are colorless flakes Obtained by microscope observation, the crystal is Ggliflozin single crystal, the yield of Ggliflozin single crystal is 84%, and the purity is 99.5%. Using X-ray powder diffractometer to detect, Empagliflozin single crystal X X-ray powder diffraction pattern as figure 1 shown.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap