Blue oligomer based on dibenzothiophene-S,S-dioxide unit and preparation method and application of blue oligomer
A technology of dioxydibenzothiophene and oligomer, which is applied in the field of blue oligomer and its preparation, can solve the problem of low performance of polymer OLED devices, high price of OLED device products, and limited market competitiveness of OLED products. and other problems, to achieve the effect of good spectral stability, novel structure and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
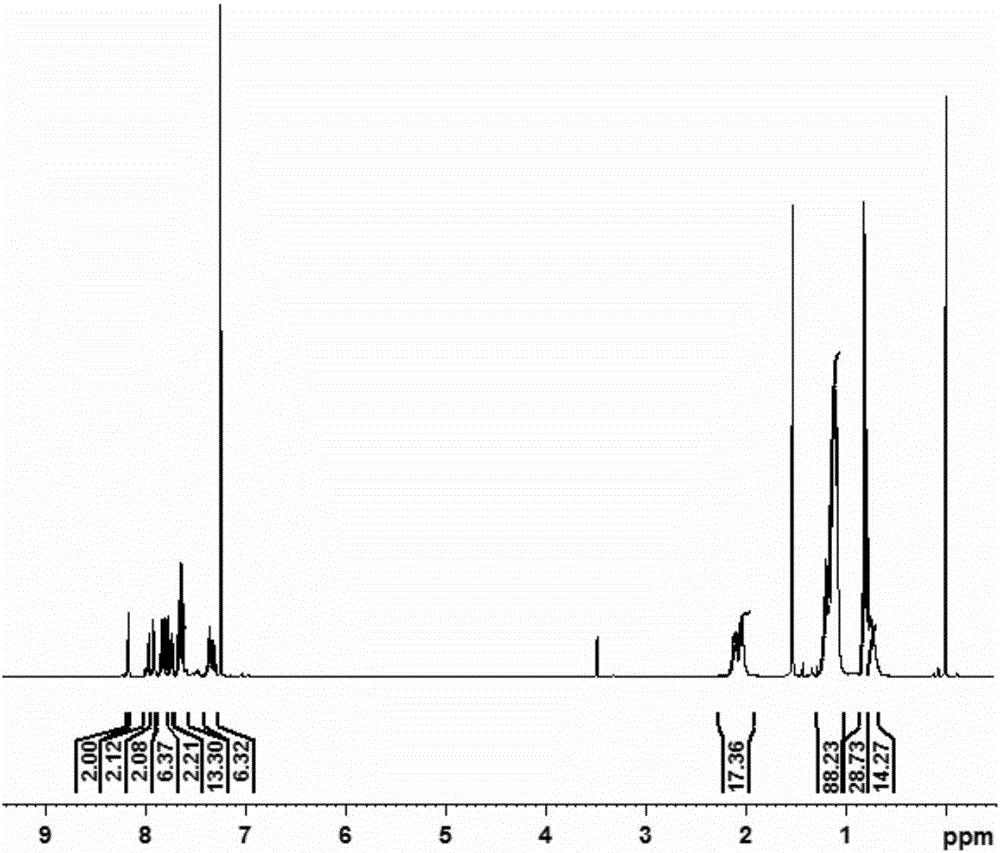
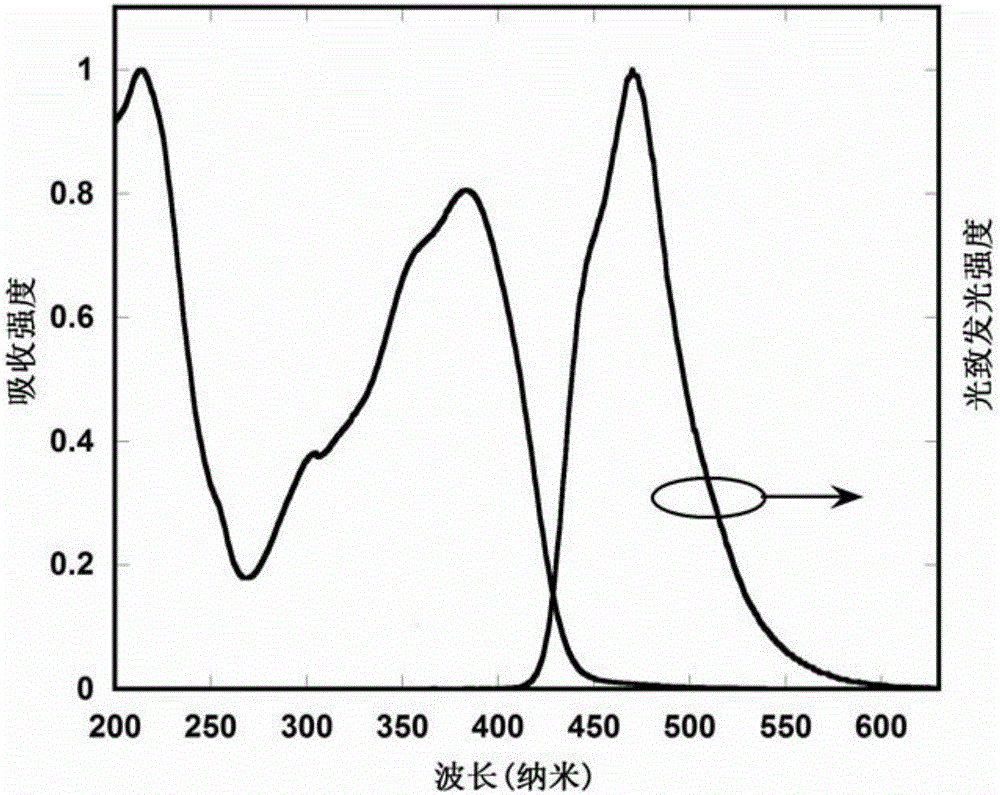
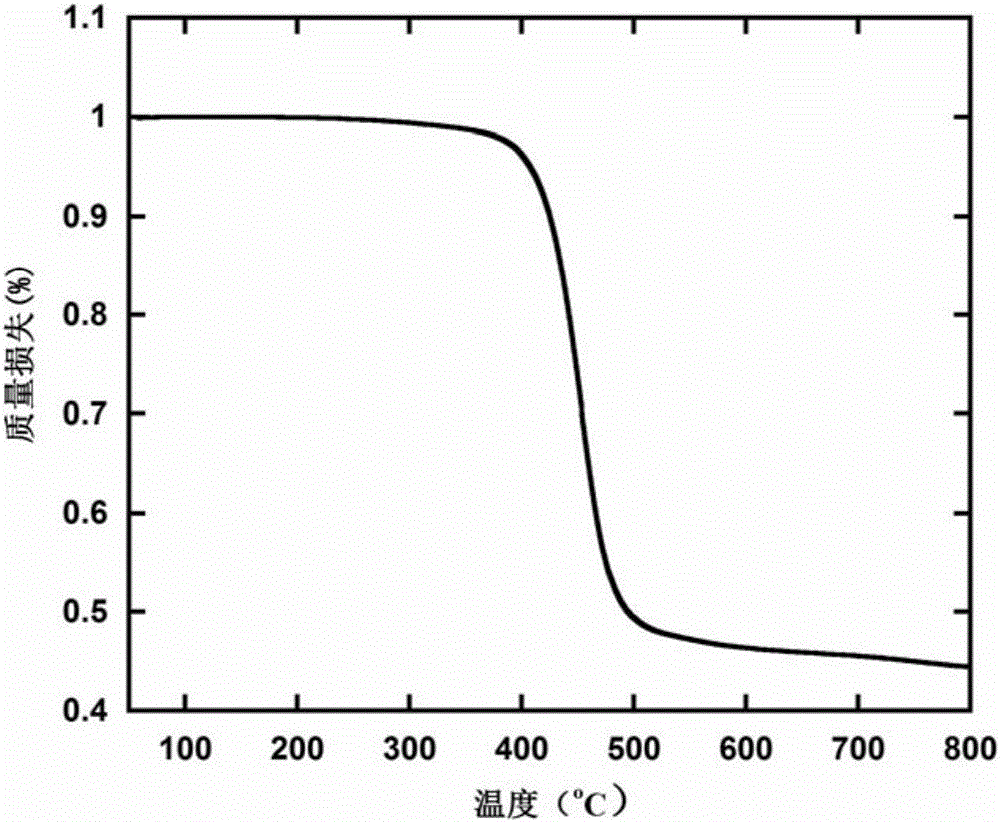
Image
Examples
Embodiment 1
[0046] Preparation of 2,7-dibromofluorene
[0047] In a 1000mL three-necked flask, add fluorene (60g, 301mmol), iron powder (0.84g, 15mmol) and chloroform (400mL), ice-bath to 5°C, under dark conditions, liquid bromine (35mL, 753mmol) and 115mL The mixture of chloroform was slowly added dropwise into the reaction solution, and after the dropwise addition was completed, the mixture was vigorously stirred at room temperature (stirring speed was 800 rpm) and reacted for 12 hours. Add 200 mL of a saturated aqueous solution of sodium bisulfite to the reaction flask to quench the reaction. The reaction mixture was suction filtered, and the filter residue was washed three times successively with saturated aqueous sodium bisulfite solution, water and ethanol. After the filter residue was dried, it was washed with CHCl 3 Purification was carried out by recrystallization to obtain 77.8 g of white crystals, yield: 80%. 1 H NMR (300MHz, CDCl 3 ) (ppm): 7.54 (d, 2H), 7.44 (d, 2H), 7.31 ...
Embodiment 2
[0050] Preparation of 2,7-dibromo-9,9-dioctylfluorene
[0051]Under the protection of argon, in a 500mL three-necked flask, add 2,7-dibromofluorene (32.4g, 100mmol) and dimethyl sulfoxide (250mL), under vigorous stirring (stirring speed is 800rpm), add tetrabutyl Ammonium bromide (1.61g, 5mmol), and then sodium hydroxide (40g, 1mol) 50wt% aqueous solution was slowly added dropwise, and the reaction was completed for 2 hours, and 1-bromooctane (57.9g, 0.3mol) was injected in one go. After continuing to react for 10 hours, stop the reaction, pour the reaction solution into water, add aqueous hydrochloric acid for neutralization, extract with dichloromethane, wash 7 times with saturated brine, dry, spin dry the solvent, and use column chromatography to analyze the crude The product was purified by using petroleum ether as an eluent to obtain 43.8 g of a white solid with a yield of about 80%. 1 H NMR (300MHz, CDCl 3 )(ppm): 7.53(d, 2H), 7.46(d, 2H), 7.41(d, 2H), 1.94(m, 4H), 1.2...
Embodiment 3
[0054] Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9,9-dioctylfluorene
[0055] Under the protection of argon, add 2,7-dibromo-9,9-dioctylfluorene (21.9g, 40mmol) and 250mL of anhydrous tetrahydrofuran into a 500mL three-neck flask, and cool the reaction liquid to -78°C with liquid nitrogen. ℃, slowly drop n-butyllithium in n-hexane solution (48mL, 2.5M, 120mmol), keep stirring at -78℃ for 2 hours, inject 2-isopropyl-4,4,5,5-tetra Methyl-1,3,2-dioxaborane (26g, 140mmol), let it rise to room temperature naturally, and continue to react for 20h. Add ammonium chloride aqueous solution to quench the reaction, rotary evaporate most of the solvent, the reaction mixture is poured into water, and extracted with dichloromethane, washed 5 times, the organic phase is separated, dried, and after filtering and spin-drying the solvent, the column chromatography method ( Eluent: petroleum ether) was used to purify the crude product, and 19.8 g of white solid was obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com