Aggregation-induced light emission red delay material, preparation method and application thereof
An aggregation-induced luminescence, red technology, applied in the direction of luminescent materials, preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of fluorescence quenching, device efficiency roll-off, long fluorescence lifetime, etc., achieve short synthesis route, reduce Lighting up voltage, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of 2-carbazol-9-yl-anthraquinone
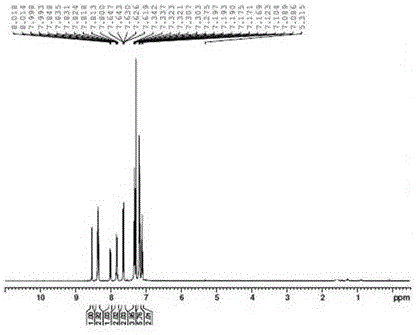
[0045]In a 100mL three-necked flask, add 2-bromoanthraquinone (3.45g, 12mmol), carbazole (2.00g, 12mmol), cuprous iodide (0.23g, 1.2mmol), 1,10-phenanthroline monohydrate (0.24g, 1.2mmol), anhydrous potassium carbonate (2.45g, 18mmol), and 18.5mL N,N-dimethylformamide were mixed evenly, and refluxed for 20 hours under nitrogen protection. The reaction solution was cooled to room temperature, and 50mL water, and then extracted with dichloromethane (2*100 mL), the organic layers were combined, dried over anhydrous sodium sulfate and concentrated to give a brown oil. After column chromatography, eluting with a mixture of dichloromethane and petroleum ether at a volume ratio of 1:5, a red solid 2-carbazol-9-yl-anthraquinone was obtained, and the prepared 2-carbazole-9 The yield of -yl-anthraquinone is 65.7%. 1 H NMR (300MHz, CDCl 3 )δ, (ppm): 8.56-8.76 (d, 2H), 8.37-8.46 (t, 2H), 8.17-8.19 (d, 2H), 8.07-8.10 (m, 1H), 7.86-7....
Embodiment 2
[0047] Preparation of 2-carbazol-9-yl-anthraquinone
[0048] In a 100mL three-necked flask, add 2-bromoanthraquinone (3.45g, 12mmol), carbazole (2.00g, 12mmol), cuprous iodide (0.35g, 1.83mmol), 1,10-phenanthroline monohydrate (0.35g, 1.77mmol), anhydrous potassium carbonate (3.28g, 24mmol), 40mLN, N-dimethylformamide were mixed uniformly, and reflux reaction was carried out under nitrogen protection for 24 hours, the reaction solution was cooled to room temperature, and 50mL of water was added, Then it was extracted with ethyl acetate (2*100 mL), the organic layers were combined, dried over anhydrous sodium sulfate and concentrated to obtain a brown oil. After column chromatography, eluting with a mixture of dichloromethane and petroleum ether at a volume ratio of 1:10, a red solid 2-carbazol-9-yl-anthraquinone was obtained, and the prepared 2-carbazole-9 The yield of -yl-anthraquinone was 72.3%. 1 H NMR (300MHz, CDCl 3 )δ, (ppm): 8.56-8.76 (d, 2H), 8.37-8.46 (t, 2H), 8.17...
Embodiment 3
[0050] In a 100mL three-necked flask, add 2-bromoanthraquinone (3.45g, 12mmol), carbazole (2.40g, 14.4mmol), cuprous iodide (0.46g, 2.4mmol), 1,10-phenanthroline monohydrate Compound (0.484g, 2.4mmol), anhydrous potassium carbonate (3.28g, 24mmol), 46mL N, N-dimethylformamide were mixed evenly, and the reaction was refluxed under nitrogen protection for 40 hours. The reaction solution was cooled to room temperature, 50 mL of water was added, and then extracted with dichloromethane (2*100 mL), the organic layers were combined, dried over anhydrous sodium sulfate and concentrated to obtain a brown oil. After column chromatography, eluting with a mixture of dichloromethane and petroleum ether at a volume ratio of 1:20, a red solid 2-carbazol-9-yl-anthraquinone was obtained, and the prepared 2-carbazole-9 The yield of -yl-anthraquinone was 68.7%. 1 H NMR (300MHz, CDCl 3 )δ, (ppm): 8.56-8.76 (d, 2H), 8.37-8.46 (t, 2H), 8.17-8.19 (d, 2H), 8.07-8.10 (m, 1H), 7.86-7.91 (m, 2H ), 7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com