Method for improving heat stability of creatinase
A thermal stability, creatinase technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of poor stability, large amount of creatinase, no chemical bond formation, etc., and achieves convenient application, improved thermal stability, and thermal stability. Sex-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the construction of mutant strain
[0026] (1) Chemically synthesize the CRE gene fragment, whose sequence is shown in GenBank accession number A10619.1 (creatinase derived from Pseudomonasputida), connect the gene fragment to pET28a(+), transform E.coli, and obtain the expression wild enzyme Recombinant E. coli. Extract the recombinant E.coli and the plasmid, and verify that the correct plasmid is the recombinant plasmid pET28a(+)-CRE.
[0027] (2) Using pET28a(+)-CRE as a template, carry out site-directed mutagenesis by overlapping extension PCR technology, and then digest and connect to pET28a(+). The mutant plasmid pET28a(+)-H125C / L130C with amino acid substitutions at positions 125 and 130 of cholesterol oxidase in GenBank: CAA00921.1 was obtained.
[0028] (3) Transform pET28a(+)-H125C / L130C into Escherichia coli E.coli BL21(DE3), verify the recombinant transformants, and verify that the correct one is the mutant strain E.coli-H125C / L130C. Creatin...
Embodiment 2
[0029] Embodiment 2: Purification of enzyme produced by mutant strain fermentation
[0030] (1) Preparation and purification of crude enzyme solution
[0031] The culture conditions of the seed liquid: use 250mL shake flask culture, the filling liquid is 20% LB medium, and add filter-sterilized 100mg·mL in the medium -1 Take 50 μL of kanamycin sulfate, take a single colony into the culture medium, and culture overnight at 37°C and 200 rpm.
[0032] Fermentation broth culture conditions: cultured in 500mL shake flasks, filled with 20% LB medium, and added filter-sterilized 100mg·mL -1 Add 100 μL of kanamycin sulfate, add 5% seed solution, cultivate at 37°C, 200 rpm until the OD reaches 0.6-0.8, add IPTG with a final concentration of 1 mM, induce culture for 16 hours at 16°C, 200 rpm.
[0033] Collection of bacteria and obtaining crude enzyme solution: centrifuge the fermentation broth at 8000rpm for 5min, weigh the wet weight, add 20mL pH7.5, 50mmol·mL according to 1g of wet ...
Embodiment 3
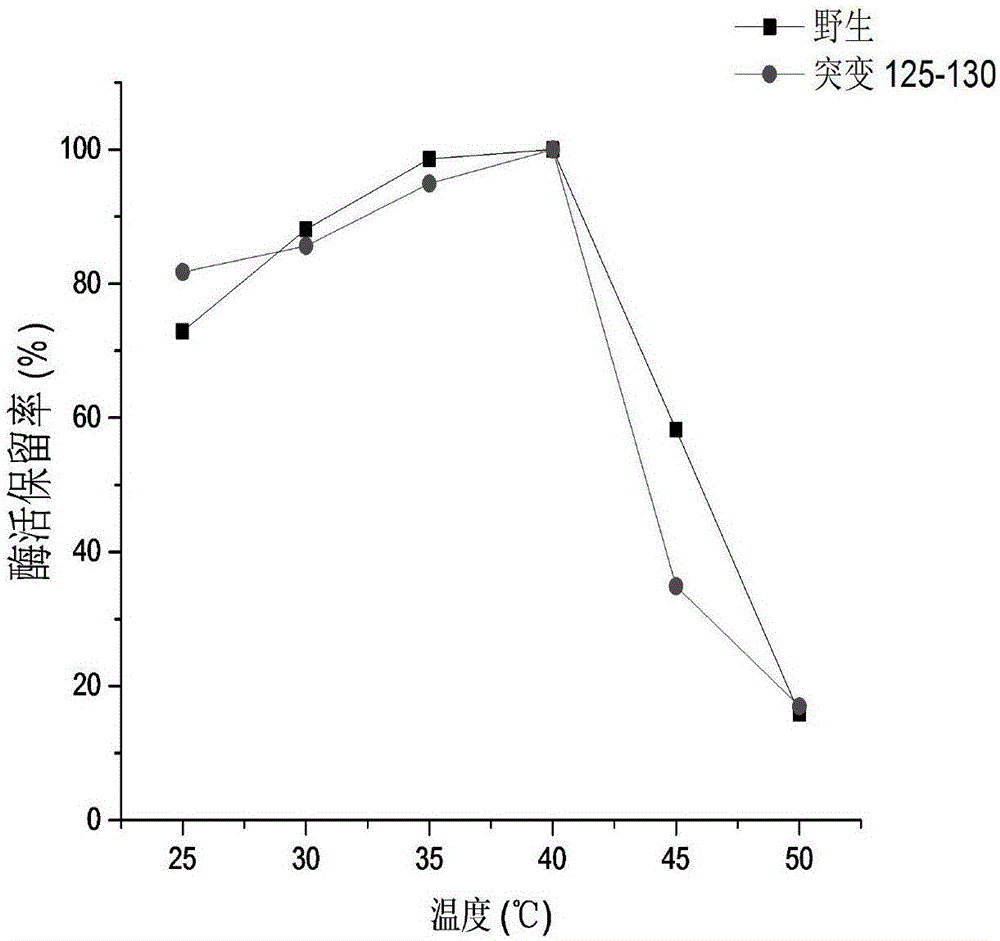
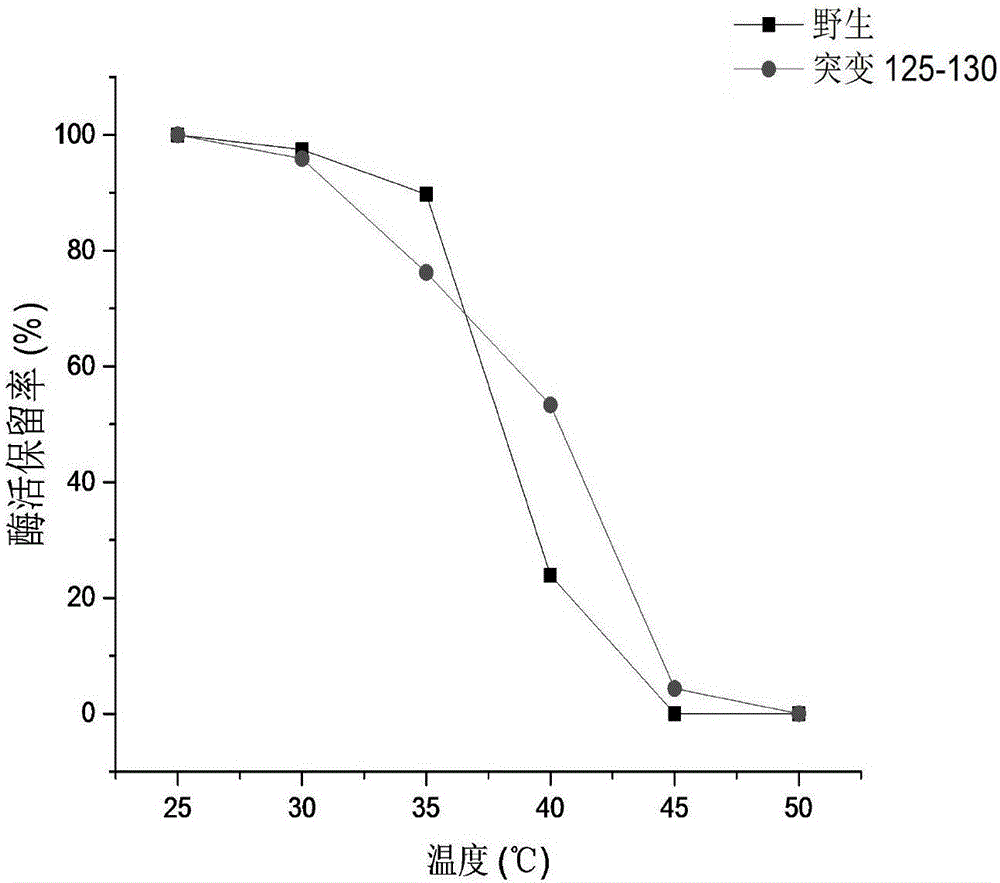
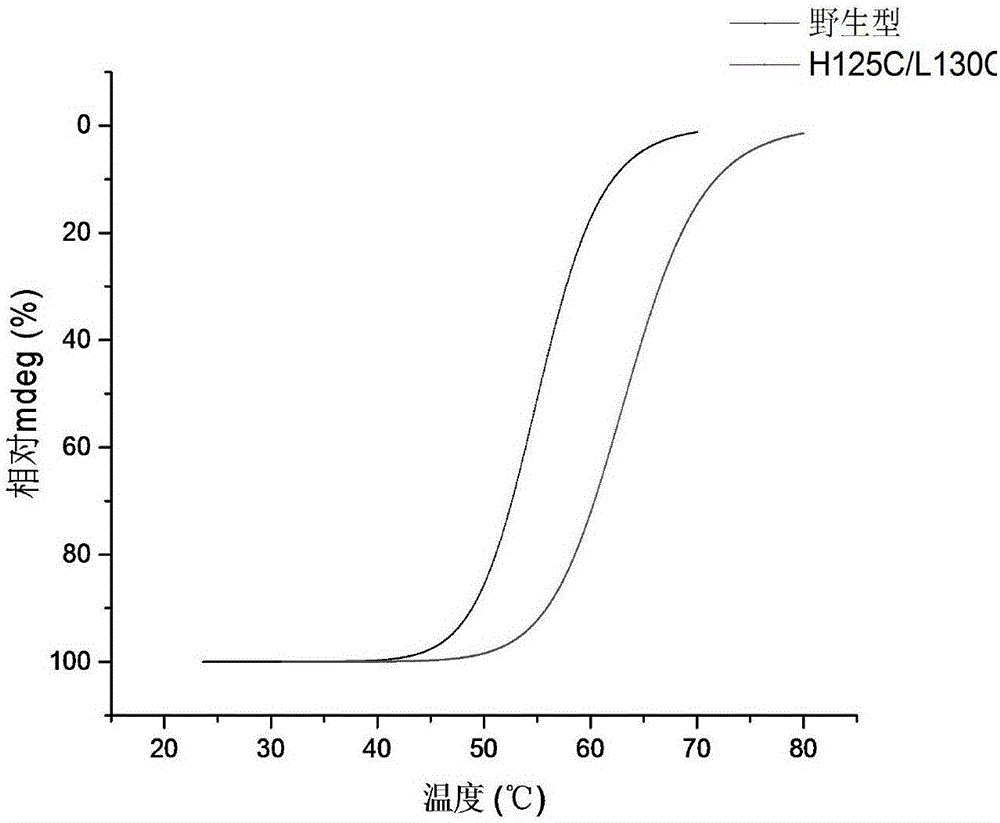
[0035] Example 3: Activity determination of CRE and thermal stability comparison of creatinase before and after mutation
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com