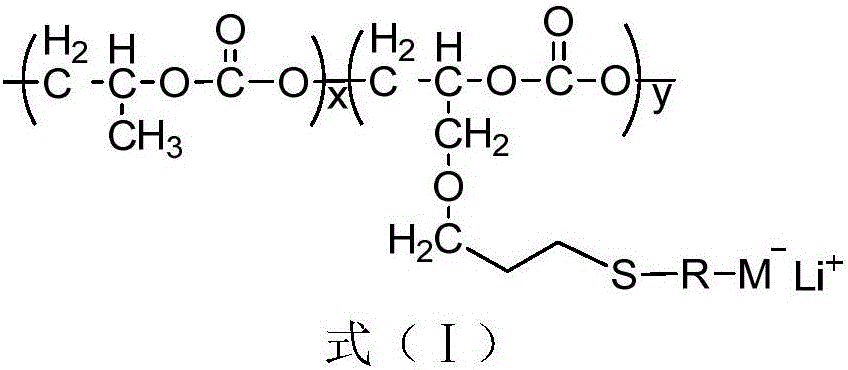
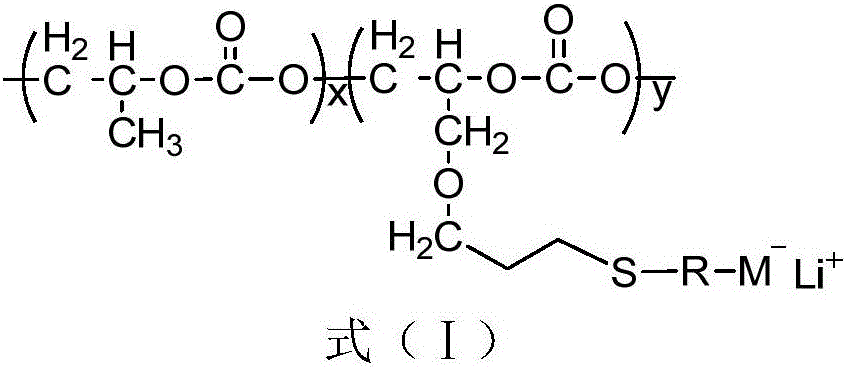
Single-lithium-ion-conducting solid polymer electrolyte adopting carbon dioxide based polycarbonate as main chain and preparation method of single-lithium-ion-conducting solid polymer electrolyte
A technology based on polycarbonate and solid polymer, which is applied in the field of organic polymer functional materials and electrochemistry, can solve the problems of low ionic conductivity of single ion conductors, complicated synthesis process, etc., and achieves simple and easy synthesis and glass transition temperature. Low cost, easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: M - Li + Preparation of an electrolyte (electrolyte 1) that is COOLi and has a molar percentage of 41.0%
[0025] Put 1.00 g of zinc glutarate catalyst (ZnGA) in the autoclave, vacuum-dry it at 80 °C for 6 h, then cool the autoclave to below 35 °C, and quickly inhale 12.9 g (0.222 mol) PO and 91.3 g ( 0.780mol) AGE, filled with CO 2 to 3.0MPa. Stir at a speed of 110r / min, open the inlet valve when the temperature rises to 80°C, and adjust the pressure in the kettle to 5.0MPa. Cool to room temperature after reacting for 40 hours, release carbon dioxide, and take out the mixture product in the still to weigh. Dissolve the product in 200mL of dichloromethane, add 0.5% BHT, and add 5% dilute hydrochloric acid to remove the catalyst, slowly pour it into frozen ethanol for precipitation under mechanical stirring, repeat the above process to completely remove the monomer and catalyst, After that, it was dried under high vacuum at 80° C. for 20 hours to obtain P...
Embodiment 2
[0029] Example 2: M - Li + Preparation of COOLi and 24.0% electrolyte (electrolyte 2) by mole percent
[0030] The polymer electrolyte was prepared in the same manner as in Example 1, except that the feed was changed to PO 25.6g (0.441mol) and AGE 75.3g (0.660mol).
Embodiment 3
[0031] Example 3: M - Li + The preparation of the electrolyte (electrolyte 3) that is COOLi and the molar percentage is 13.4%
[0032] The polymer electrolyte was prepared in the same manner as in Example 1, except that the feed was changed to PO 42.0 g (0.723 mol) and AGE 54.8 g (0.480 mol).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com