Tumor-targeting cell drug carrier and application thereof
A tumor targeting and drug technology, applied in the field of biomedicine, can solve the problems such as charging properties, hydrophilicity and hydrophobicity affecting the drug loading rate of cell carriers, carrier cells cannot achieve long-term in vivo circulation, and limiting the application significance of cell drug carriers, etc. Easy to purify, easy to obtain, simple to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] [Example 1] Synthesis of Embedded Cell Membrane Bonding Compound
[0056] Cell-embedded membrane bond (palmitic acid-lysine (camptothecin)-arginine-arginine-arginine-arginine), (palmitic acid-lysine (protoporphyrin)-arginine Synthesis of acid-arginine-arginine-arginine) and (palmitic acid-lysine (fluorescein)-arginine-arginine-arginine-arginine):
[0057] (1) Add 0.5 g of chlorine resin (1.01 mmol / g) to a reactor containing 10 mL of double-distilled N,N-dimethylformamide, and wait for the chlorine resin to dissolve in N,N-dimethylformamide N,N-dimethylformamide was withdrawn after 2 hours of swelling.
[0058] (2) Dissolve FMOC-protected amino group and Pbf-protected guanidino arginine (4 times equivalent of resin active site), N,N-diisopropylethylamine (8 times equivalent of amino acid) in 10 mL N,N - in dimethylformamide, and then put into the reactor at room temperature to react for 2 hours to bond cysteine to the resin, extract the solvent, and wash with N,N-dim...
Embodiment 2
[0070] [Example 2] Embedded cell membrane camptothecin bonded macrophage membrane and its stability over time
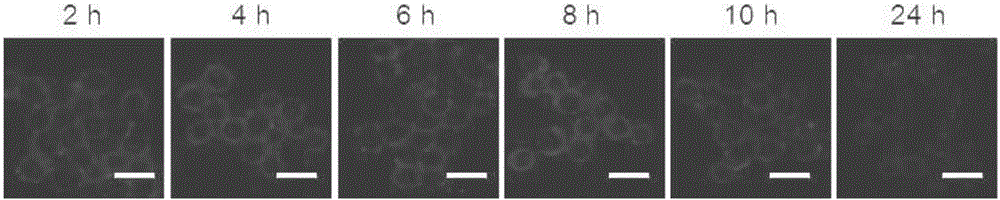
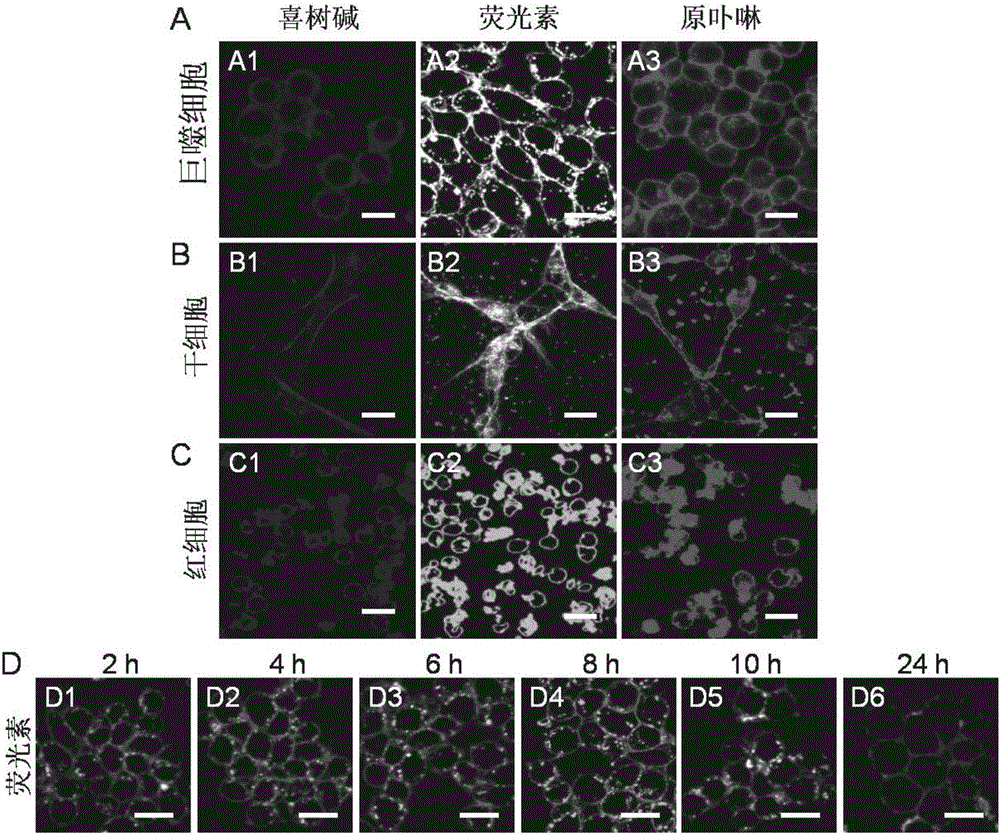
[0071] Macrophages were treated at 1×10 5 Cells / well were seeded at a density of 37°C in 1 mL of culture medium. After 24 hours, the cell-embedded camptothecin conjugate was dissolved in the medium, and 1 mL of the medium containing the cell-embedded camptothecin conjugate (30 μmol / L) was added to the macrophages. After culturing for 2 hours, aspirate the culture medium containing the camptothecin bond embedded in the cell membrane, wash the cells three times with PBS buffer solution, add new medium, and observe the fluorescence intensity of camptothecin in the cells with a laser confocal microscope .
[0072] The result is as figure 1 As shown, the embedded cell membrane camptothecin conjugate can be effectively embedded in the macrophage cell membrane. At the same time, the fluorescence intensity on the cell membrane surface is only weakly reduced when the cultu...
Embodiment 3
[0073] [Example 3] Embedded cell membrane protoporphyrin bonded macrophage and its stability over time
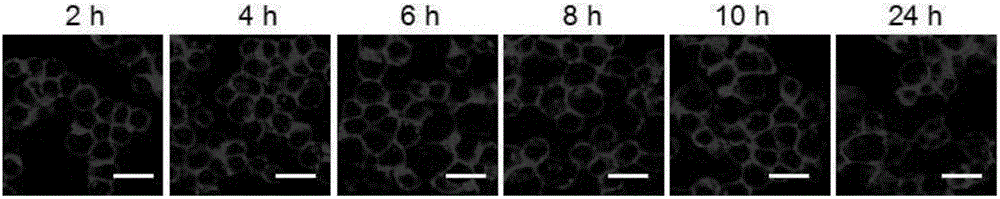
[0074] Macrophages were treated at 1×10 5 Cells / well were seeded at a density of 37°C in 1 mL of culture medium. After 24 hours, the cell-embedded membrane protoporphyrin conjugate was dissolved in the medium, and 1 mL of the medium containing the cell-embedded membrane protoporphyrin conjugate (30 μmol / liter) was added to the macrophages. After culturing for 2 hours, suck out the medium containing protoporphyrin bonds embedded in the cell membrane, wash the cells three times with PBS buffer solution, add new medium, and observe the fluorescence intensity of protoporphyrin in the cells with a laser confocal microscope .
[0075] The result is as figure 2 As shown, the cell-embedded protoporphyrin bond can be effectively embedded in the macrophage cell membrane, and at the same time, the fluorescence intensity on the cell membrane surface is only weakly reduced as the cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com