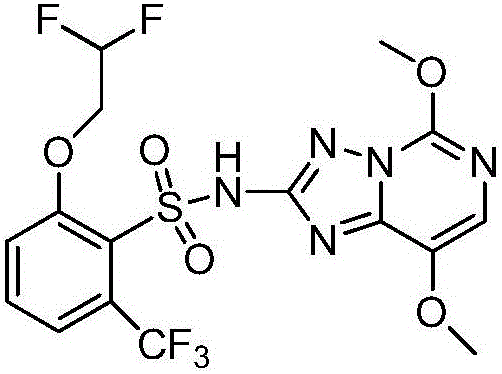
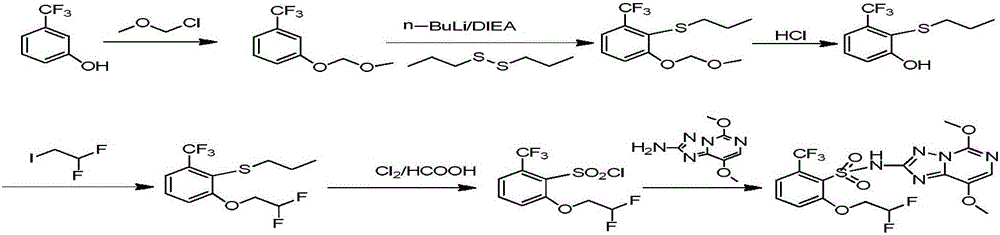
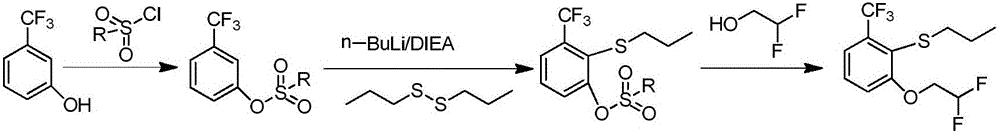
Method for preparing penoxsulam intermediate
A technology for penoxsulam and intermediates, which is applied in the field of preparation of penoxsulam intermediates, can solve the problems of difficult preparation, high price, and low industrial value, and achieve good reaction selectivity and reduce the amount of waste water , The effect of synthetic route safety and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Get a 2000mL four-port reactor, put a stirring bar, add 180g m-trifluoromethylphenol, add 800mL methylene chloride (ρ=1.325g / mL), add 178mL of tetramethylethylenediamine (ρ=0.78g / mL), stir. Keeping the temperature at -15°C, take 65mL of allyl chloroformate (ρ=1.98g / mL), slowly and continuously drop it with a constant pressure dropping funnel, and stir at room temperature for 3h. Dichloromethane was distilled off under reduced pressure, and then distilled under reduced pressure to obtain 257 g of 1-(allyloxycarbonyloxy)-3-(trifluoromethyl)benzene with a yield of 95%. H NMR spectrum: 1 H NMR (400MHz, CDCl 3 )7.70(s, 1H), 7.29~7.43(m, 3H), 6.06(m, 1H), 5.42(d, 1H), 5.28(d, 1H), 4.58(d, 2H); ESI-MS: 247.0[ M+H + ]
[0043]Take a 2000mL three-necked flask and protect it with nitrogen, and dissolve 246g of 1-(allyloxycarbonyloxy)-3-(trifluoromethyl)benzene in 1000mL of THF (tetrahydrofuran ρ=0.888g / mL). Another 165 mL of tetramethylethylenediamine (p=0.78 g / mL) and 7.5...
Embodiment 2
[0048] Take a 2000mL four-port reactor, put a stirring bar, add 180g m-trifluoromethylphenol, add 800mL chloroform (ρ=1.50g / mL), add 187g of pyridine (ρ=0.98g / mL), and stir . Keeping the temperature at -35°C, take 65mL of allyl chloroformate (ρ=1.98g / mL), slowly add it dropwise in batches with a constant pressure dropping funnel, and stir at room temperature for 6h. Chloroform was distilled off under reduced pressure, and then distilled under reduced pressure to obtain 260 g of 1-(allyloxycarbonyloxy)-3-(trifluoromethyl)benzene with a yield of 95.1%. H NMR spectrum: 1 H NMR (400MHz, CDCl 3 )7.70(s, 1H), 7.29~7.43(m, 3H), 6.06(m, 1H), 5.42(d, 1H), 5.28(d, 1H), 4.58(d, 2H); ESI-MS: 247.0[ M+H + ].
[0049] Take a 2000mL three-necked flask and protect it with nitrogen, and dissolve 246g of 1-(allyloxycarbonyloxy)-3-(trifluoromethyl)benzene in 1000mL of THF (tetrahydrofuran ρ=0.888g / mL). Another 165 mL of triethylamine (p=0.73 g / mL) and 7.5 mL of diisopropylamine were added....
Embodiment 3
[0054] Get 2000mL four-hole reactor, put into stirring bar, add 180g m-trifluoromethylphenol, add the mixture of 800mL dichloromethane (ρ=1.326g / mL) and trichloromethane (ρ=1.50g / mL), Add 230 g of triethylamine (ρ=0.728 g / mL), and stir. Keep the temperature at -35°C under the acetone liquid nitrogen system, take 65mL of allyl chloroformate, slowly and continuously drop it with a constant pressure dropping funnel, and stir at room temperature for 8h. Dichloromethane and chloroform were distilled off under reduced pressure, and then distilled under reduced pressure to obtain 258 g of 1-(allyloxycarbonyloxy)-3-(trifluoromethyl)benzene with a yield of 94.9%. H NMR spectrum: 1 H NMR (400MHz, CDCl 3 )7.70(s, 1H), 7.29~7.43(m, 3H), 6.06(m, 1H), 5.42(d, 1H), 5.28(d, 1H), 4.58(d, 2H); ESI-MS: 247.0[ M+H + ].
[0055] Take a 2000mL three-necked flask, protect it with nitrogen, and dissolve 246g of 1-(allyloxycarbonyloxy)-3-(trifluoromethyl)benzene in 1000mL of THF (tetrahydrofuran ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com