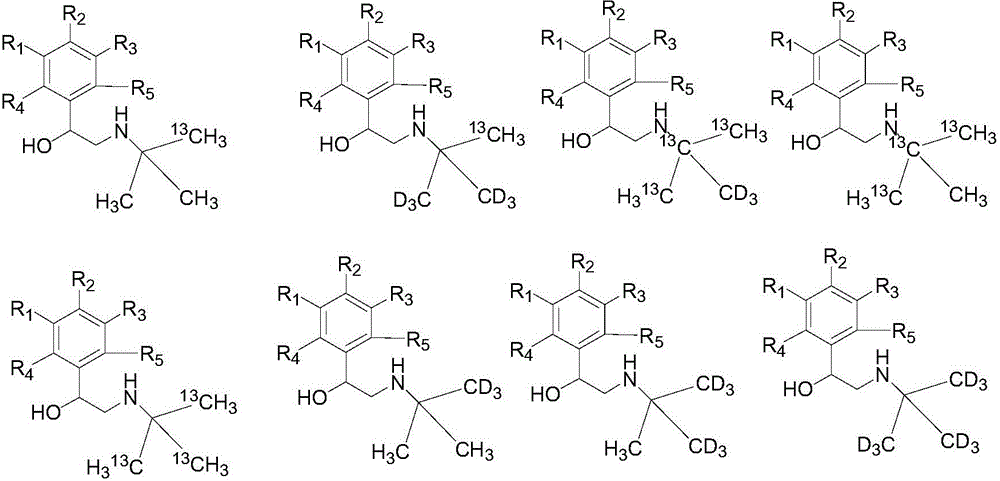
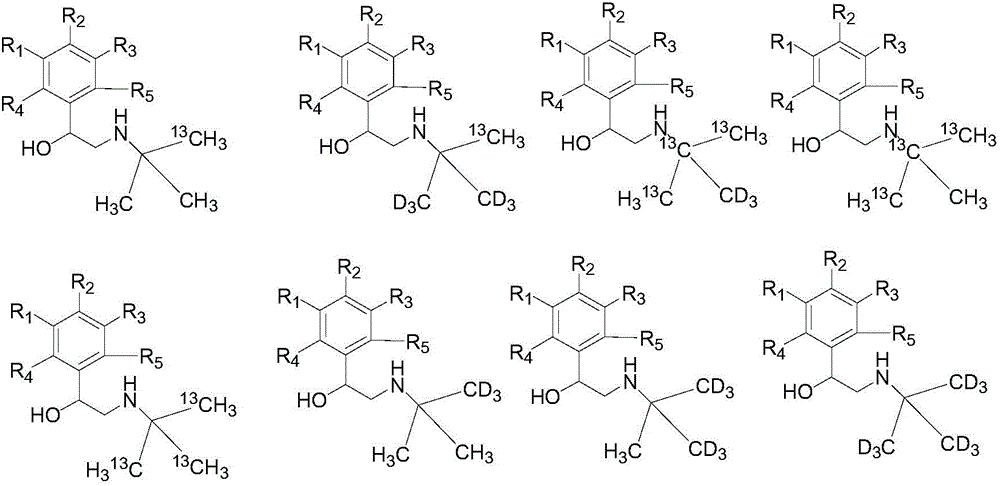
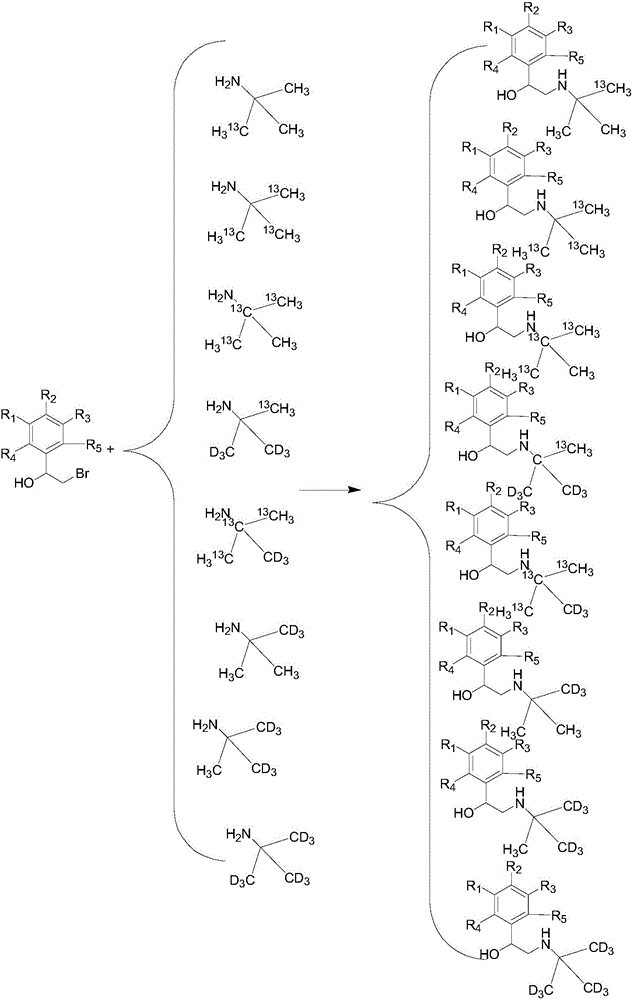
Synthesization method of stable isotope labeling beta receptor agonist
A stable isotope and synthesis method technology, applied in the field of synthesis of stable isotope-labeled β-receptor agonists, can solve the problems of difficult control of isotope abundance, low utilization rate of isotope atoms, long reaction steps, etc., and achieve high atom utilization rate , economy and use value are good, and the effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A stable isotope labeled clenbuterol- 13 Synthesis of C
[0053] Add 4.12g of 3,5-dichloro-4-aminobromophenethyl alcohol into a 35mL reaction bottle, stir and dissolve 20mL of dichloromethane, then add tert-butylamine- 13 C 1.5g, triethylamine 2g, closed system, microwave power 50 watts, pressure 50psi; reaction temperature 40°C, reaction time 5min, after the reaction was completed, washed with water, dried to obtain 5.04g clenbuterol- 13 C, the yield is based on tert-butylamine- 13 C 90.8%, HPLC detection, purity 99.5%; mass spectrometry detection, abundance 99.5atom% 13 c.
Embodiment 2
[0055] A stable isotope labeled tobuterol- 13 C 3 Synthesis
[0056] Add 9.42g of o-chlorobromophenylethanol to the 80mL reaction bottle, stir and dissolve 45mL of chloroform, then add tert-butylamine- 13 C 3 3.0g, 3.5g sodium bicarbonate, closed system, set the microwave power to 100 watts, and the pressure to 20psi; the reaction temperature was 50°C, and the reaction time was 4min. After the reaction was completed, it was washed with water and dried to obtain 8.34g tobuterol- 13 C 3 , yield in terms of tert-butylamine- 13 C 3 Total 90.4%, HPLC detection, purity 99.2%; mass spectrometry detection, abundance 99.4atom% 13 c.
Embodiment 3
[0058] A stable isotope labeled brobuterol- 13 C 3 Synthesis
[0059] Add 7.44g of 3,5-dichloro-4-aminobromophenethyl alcohol into a 35mL reaction bottle, stir and dissolve 20mL of tetrahydrofuran, then add tert-butylamine- 13 C 3 1.6g, sodium carbonate 1.1g, closed system, set the microwave power to 40 watts, and the pressure to 35psi; the reaction temperature was 65°C, and the reaction time was 3min. After the reaction was completed, it was washed with water and dried to obtain 6.73g of brobuterol- 13 C 3 , yield in terms of tert-butylamine- 13 C 3 Total 91.2%, HPLC detection, purity 99.8%; mass spectrometry detection, abundance 99.5atom% 13 c.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com