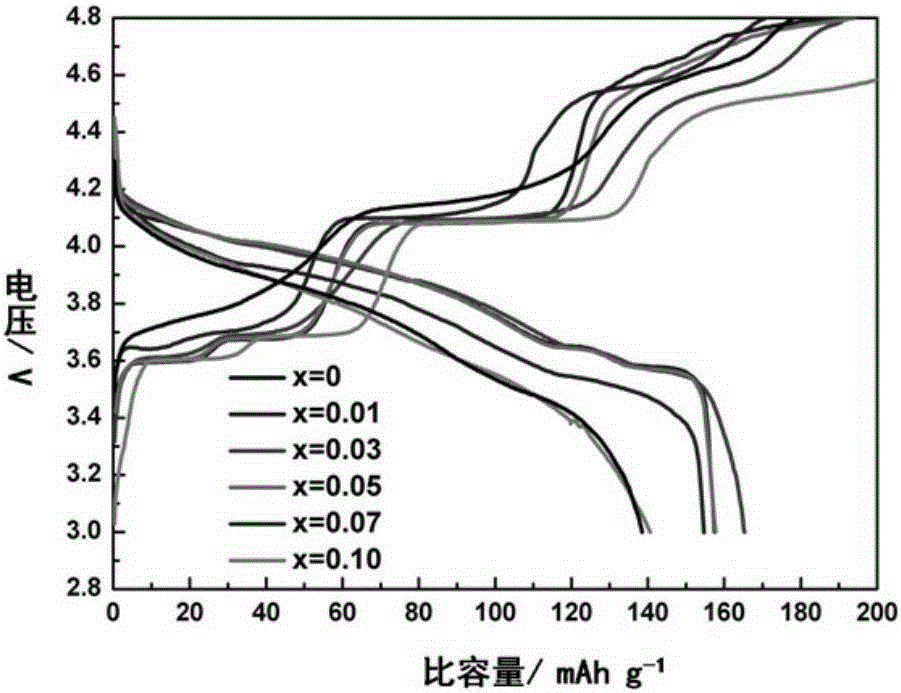
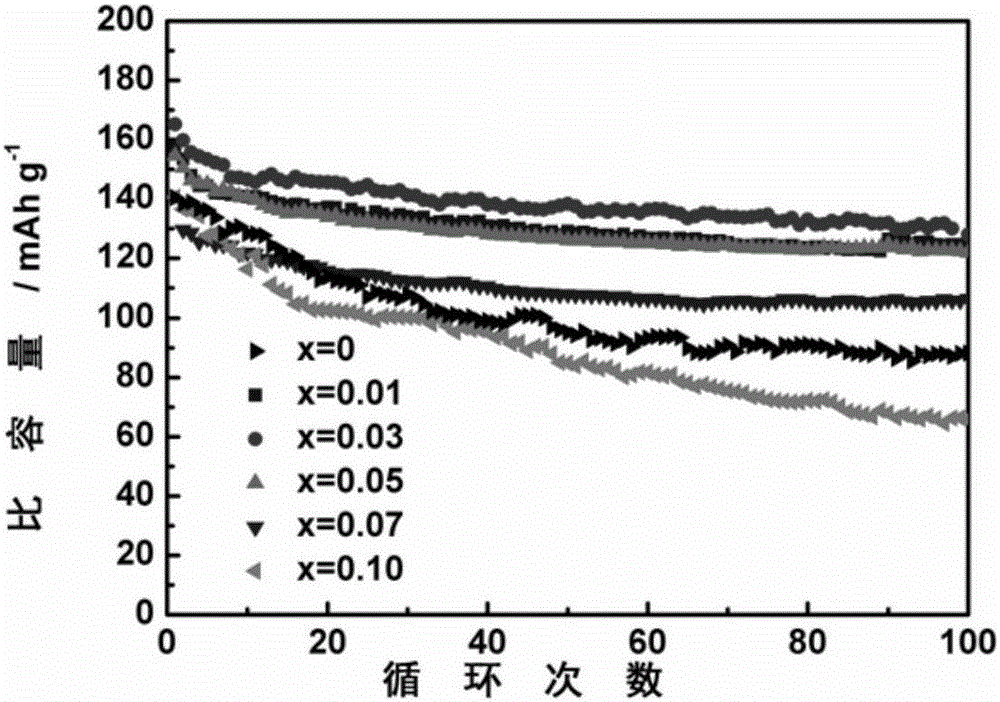
Applications of boron-doped lithium vanadium phosphate positive electrode material in lithium ion battery
A lithium-ion battery and lithium vanadium phosphate technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as low conductivity, poor high-current discharge performance, and poor high-voltage cycle stability, and achieve simple modification methods , the effect is obvious, and the effect is easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Dissolve 0.003mol of oxalic acid in a beaker filled with deionized water, then add 0.001mol of vanadium pentoxide, stir in a constant temperature water bath at 70-80°C until it turns into a blue solution, then add 0.00299mol of ammonium dihydrogen phosphate , 0.00001mol of boric acid, 0.0015mol of lithium carbonate, and continue to stir to form blue-green doped lithium vanadium phosphate sol (Li:V:B:PO 4 3- =3:2:0.01:2.99). The sol was baked in an oven at 80°C for about 10 hours to obtain a blue fluffy precursor, which was ground into powder and placed in a tube furnace, and heated at a rate of 3°C / min to 350°C and keep it warm for 4h, after cooling down, take it out and grind it, continue to raise the temperature to 750°C at a rate of 3°C / min and keep it warm for 10h, to get a lithium vanadium phosphate composite powder doped with 1% boron.
Embodiment 2
[0021] Dissolve 0.003mol of oxalic acid in a beaker filled with deionized water, then add 0.001mol of vanadium pentoxide, stir in a constant temperature water bath at 70-80°C until it turns into a blue solution, then add 0.00297mol of ammonium dihydrogen phosphate , 0.00003mol of boric acid, 0.0015mol of lithium carbonate, and continue to stir to form blue-green doped lithium vanadium phosphate sol (Li:V:B:PO 4 3- =3:2:0.03:2.97). Place the sol in an oven at 80°C and bake for about 10 hours to obtain a blue fluffy precursor. Grind the precursor into powder and place it in a tube furnace. Under an argon atmosphere, heat up at a rate of 3°C / min. Heat it to 350°C and hold it for 4 hours, take it out and grind it after cooling down, continue to heat up to 750°C at a heating rate of 3°C / min and hold it for 10 hours to obtain a lithium vanadium phosphate composite material powder doped with 3% boron.
Embodiment 3
[0023] Dissolve 0.003mol of oxalic acid in a beaker filled with deionized water, then add 0.001mol of vanadium pentoxide, stir in a constant temperature water bath at 70-80°C until it turns into a blue solution, then add 0.00295mol of ammonium dihydrogen phosphate , 0.00005mol of boric acid, 0.0015mol of lithium carbonate, and continue to stir to form blue-green doped lithium vanadium phosphate sol (Li:V:B:PO 4 3- =3:2:0.05:2.95). Place the sol in an oven at 80°C and bake for about 10 hours to obtain a blue fluffy precursor. Grind the precursor into powder and place it in a tube furnace. Under an argon atmosphere, heat up at a rate of 3°C / min. Heat it to 350°C and hold it for 4 hours. After cooling down, take it out and grind it. Continue to heat up to 750°C at a heating rate of 3°C / min and hold it for 10 hours to reach the lithium vanadium phosphate composite material powder doped with 5% boron.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com