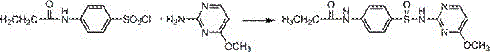Preparation method of antibacterial veterinary drug sulfamonomethoxine sodium
A technology of sulfamethoxine and sodium metmethoxine, which is applied in the field of preparation of veterinary drug sulfamethoxine sodium, can solve the problems of low yield, cumbersome production process operation, long reaction time, etc., and achieve low cost , The preparation process is simple and the effect of reducing the probability of hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: (1) Add 12.5g of 4-methoxy-2-aminopyrimidine and 7.9g of anhydrous pyridine into a reactor with a stirring device, heat the temperature to 75°C, and fully stir until 4-methoxy -2-Aminopyrimidine was fully dissolved, and then, under stirring, 0.555 g of anhydrous calcium chloride was added, and then 46.8 g of acetaminobenzenesulfonyl chloride was added to the reactor in batches within 1 hour. , keep the reaction temperature at 75°C, continue the reaction for 4 hours, stop the reaction and filter, pour the crude reaction product into hot water with 4 times the amount of pyridine, stir for 1 hour, refine for 3 hours, filter, wash the filter cake with water until the filtrate is clear, and dry Dry to constant weight to obtain 27.5 g of sulfamethoxine compound with a yield of 98.2% and a purity of 99.5%.
[0027] (2) Dissolve 27.5g of sulfamethoxine in water, heat and stir, heat to 60°C, slowly add 10% sodium hydroxide solution, adjust the pH to 12-13, stir until ...
Embodiment 2
[0028] Example 2: Other reaction conditions remain unchanged, the reaction temperature of step (1) in Example 1 is adjusted to 65°C, and the reaction time is adjusted to 4.5h to obtain 27.4g of sulfamethoxine compound with a yield of 98.0% , with a purity of 99.5%; step (2) with the same reaction conditions, 24.5 g of sulfamethoxine sodium was obtained with a purity of 99.8%.
Embodiment 3
[0029] Example 3: Other reaction conditions remained unchanged, the reaction temperature of step (1) in Example 1 was adjusted to 70°C, and the reaction time was adjusted to 4.5 hours to obtain 27.2 g of sulfamethoxine compound with a purity of 99.1%; Step (2) The reaction conditions were unchanged, and 24.1 g of sulfamethoxine sodium was obtained with a purity of 99.3%.
[0030]Example 3: Other reaction conditions remain unchanged, the amount of the catalyst in step (1) in Example 1 is adjusted to 0.112g, and 26.9g of sulfamethoxine compound is obtained, with a yield of 96% and a purity of 99.0%; Step (2) The reaction conditions were unchanged, and 24.1 g of sulfamethoxine sodium was obtained with a purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com