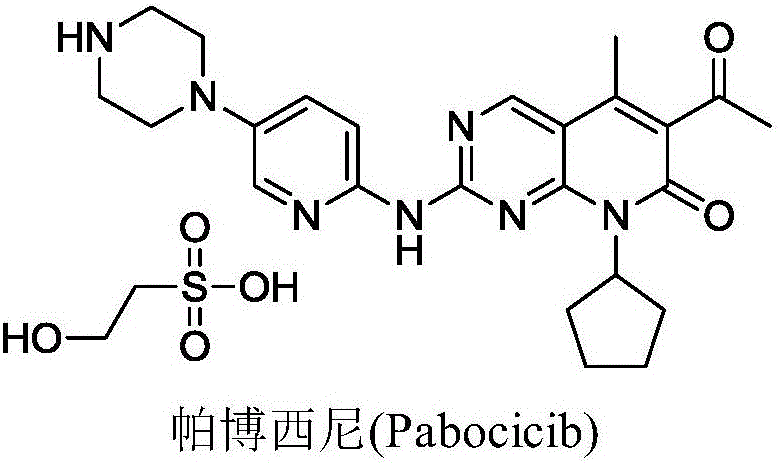
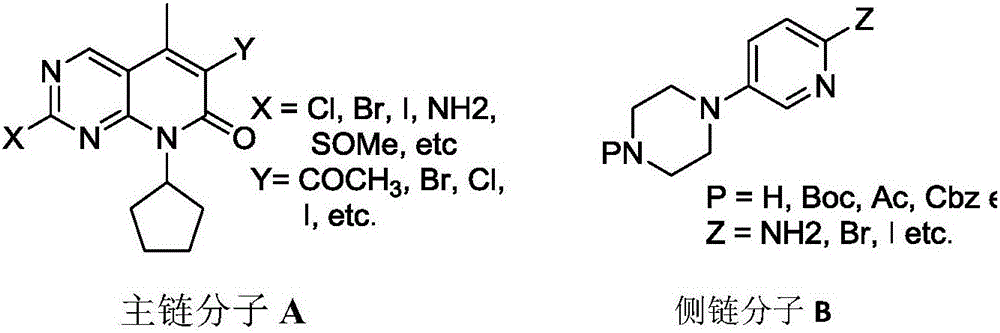
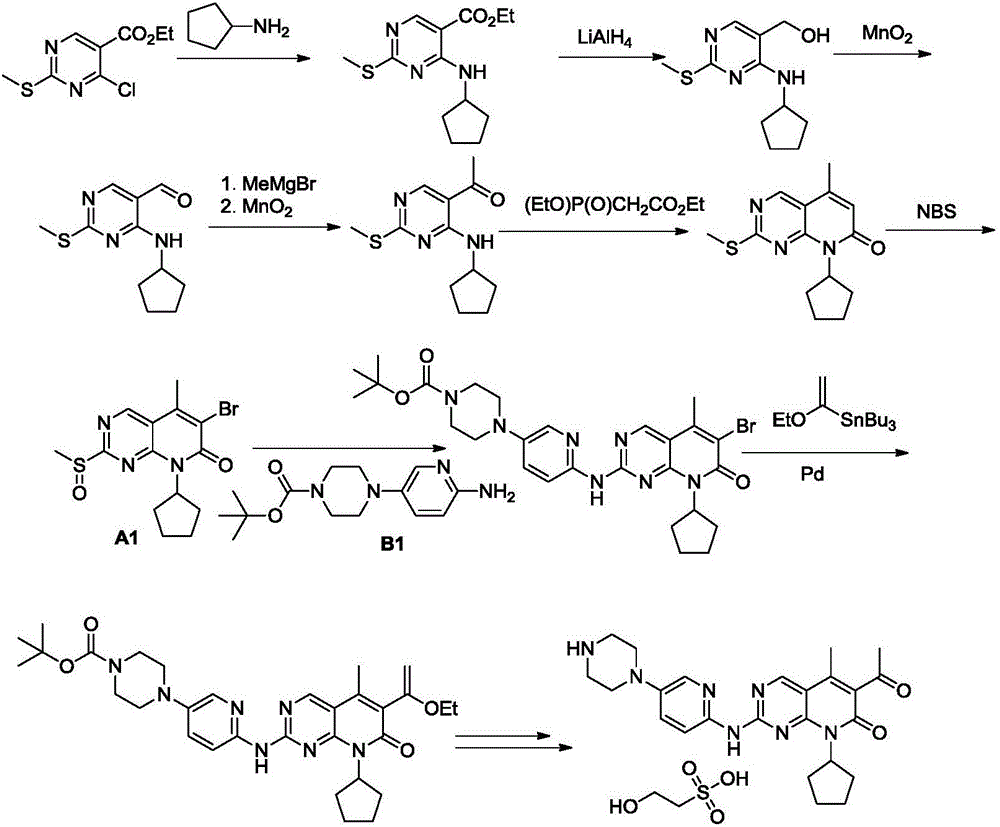
Novel synthetic method of Palbociclib
A technology of palbociclib and synthesis method, applied in the direction of organic chemistry, etc., which can solve the problems of unfavorable process amplification, difficult product purification, difficult operation, etc., and achieve the effect of avoiding coupling reaction, simple operation and shortening the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056]Add 2,4-dichloropyrimidine (14.90g, 100mmol), triethylamine (2.24g, 20mmol) and dichloromethane (149mL) into a three-necked flask, stir evenly, cool to 0-5°C, and slowly drop cyclopentyl Base amine (8.51g), after adding, warm up to room temperature and react for 6-8 hours. After the reaction, 277mL of water is added to quench the reaction. The aqueous phase is extracted 3 times with dichloromethane (75mL), and the combined organic phases are washed with saturated brine (75mL). Once, dried over sodium sulfate, concentrated and separated by column chromatography with a mixed solvent of dichloromethane and ethyl acetate to obtain compound II (15.22 g, 77%). ESI m / z=198.1(M+1).
[0057] Triethylamine in Example 1 can be replaced by diisopropylethylamine, DABCO, DBU, etc.; solvent methylene chloride can be dimethylformamide, dimethylacetamide, dimethyl sulfoxide, ethyl acetate, tetrahydrofuran , 1,4-dioxane, acetonitrile or toluene instead.
Embodiment 2
[0059]
[0060] Add 2-chloro-4-(cyclopentylamino)pyrimidine II (19.77g, 100mmol) and 1,2-dichloroethane (99mL) into the three-necked flask, stir well and cool to 0~5℃, add 2mol / L dichloromethane solution of boron trichloride (60mL, 120mmol), after the addition, keep stirring for 30 minutes, add acetonitrile (4.93g, 120mmol) dropwise, heat up to reflux reaction for 20-24 hours after the addition, finish cooling and add 4mol / L hydrochloric acid (75mL, 300mmol) quenched the reaction, and after stirring for 20 minutes, the organic phase was separated, the aqueous phase was extracted twice with dichloromethane (100mL), and the combined organic phase was washed once with saturated sodium bicarbonate (100mL) solution. Washed twice with saturated brine (100 mL), dried over sodium sulfate, concentrated and separated by column chromatography with a mixed solvent of dichloromethane and ethyl acetate to obtain compound III (21.09 g, 88%). ESI m / z=240.2(M+1).
[0061] In embodiment 2,...
Embodiment 3
[0063]
[0064] Add phosphine reagent 4 (33.34g, 110mmol) and dimethylformamide (96mL) into the three-necked flask, stir and dissolve, add potassium tert-butoxide (22.44g, 200mmol), keep warm for 30 minutes after adding, and then drop into the middle Compound III (23.97g, 100mmol) in dimethylformamide (48mL) solution, raised to room temperature after dropwise reaction for 3-4 hours. Add water 240mL to quench the reaction, the aqueous phase is extracted 3 times with ethyl acetate (120mL), the combined organic phase is washed 2 times with saturated brine (120mL), dried over sodium sulfate, and concentrated with petroleum ether ethyl acetate mixed solvent column chromatography Compound 5 (27.41 g, 80%) was isolated. ESI m / z = 342.0, 344.1 (M+1).
[0065] Potassium tert-butoxide can be replaced by sodium hydride, sodium tert-butoxide, lithium hexamethylsilamide, butyllithium or lithium diisopropylamide in embodiment 3; solvent dimethylformamide can be replaced by tetrahydrofur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com