Silybin nanometer suspension and preparation method thereof
A nano-suspension and silibinin technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of wide particle size distribution, difficult promotion and use, and grinding Solve problems such as medium dissolution, achieve the effect of increasing solubility and dissolution rate, simple prescription and preparation process, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
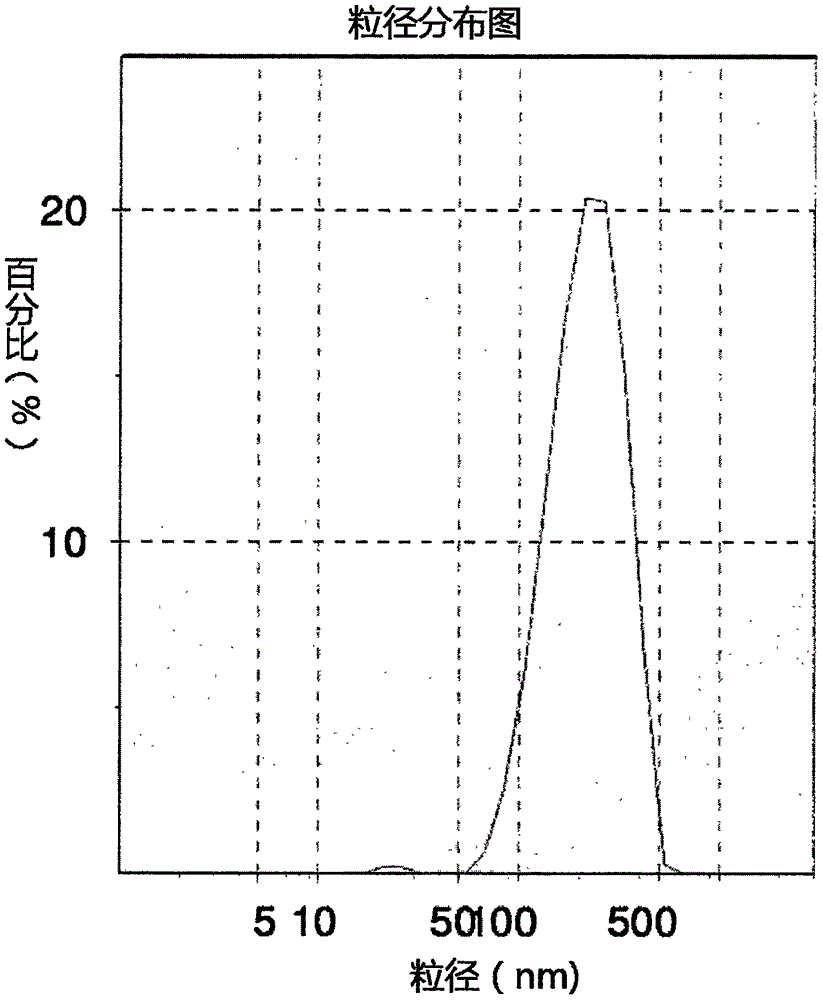
[0025] Weigh 0.010g sodium lauryl sulfate and 0.350g hydroxypropyl cellulose, put them in a beaker, add about 7g water to dissolve them completely, and make an aqueous stabilizer solution. Weigh 1.5g of silybin raw material, put it in a grinding tank, transfer all the stabilizer aqueous solution in the beaker to the grinding tank, add a small amount of water to rinse, and finally add water to 10g. Place the grinding jar in an ultrasonic cleaner for ultrasonic dispersion for 10 min. Add about 60g of zirconia grinding beads (the mass ratio of the large and small balls is 1:5, the diameter of the large ball is 5mm, and the diameter of the small ball is 0.5mm) into the grinding jar, and balance the grinding jar in pairs on the balance, and then install the grinding jar In a planetary ball mill, grind at 300r / min for 3h to obtain silibinin nano-suspension. The silibinin nano-suspension prepared by the method has an average particle diameter of 188.2nm and a PDI of 0.21.
[0026] ...
Embodiment 2
[0028] Weigh 0.1 g of sodium lauryl sulfate and 0.1 g of polyvinylpyrrolidone, put them in a beaker, add about 7 g of water to dissolve them completely, and prepare an aqueous stabilizer solution. Weigh 1.0 g of silybin raw material, put it in a grinding tank, transfer all the stabilizer aqueous solution in the beaker to the grinding tank, add a small amount of water to rinse, and finally add water to 10 g. Place the grinding jar in an ultrasonic cleaner for 10 min. Add about 50 g of zirconia grinding beads (the mass ratio of the large and small balls is 1:5, the diameter of the large ball is 5 mm, and the diameter of the small ball is 0.5 mm) into the grinding jar, and balance the grinding jar in pairs on the balance, and then install the grinding jar Grind in a planetary ball mill at 250r / min for 4h to obtain silibinin nano-suspension. The silibinin nano-suspension prepared by the method has an average particle diameter of 238.9 nm and a PDI of 0.18.
Embodiment 3
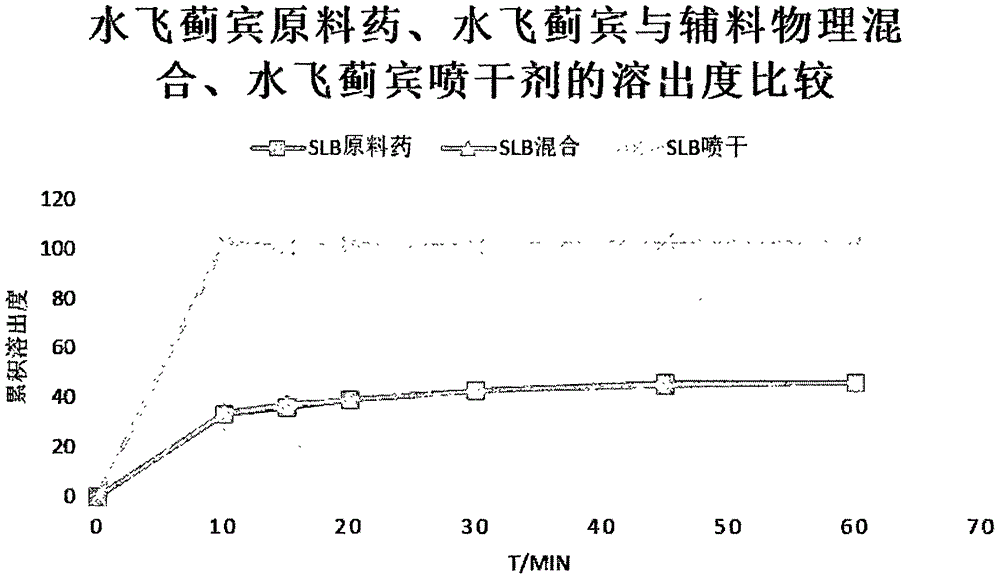
[0030] Study on the dissolution rate of silibinin nanosuspension in vitro
[0031] The silibinin raw material drug, physical mixture and the silibinin nanosuspension spray-dried powder of Example 1 of the present invention were subjected to a dissolution contrast experiment, and the samples at different time points were assayed and the cumulative dissolution percentage was calculated. The result shows that the dissolution rate of the silibinin nano-mixture spray-dried powder of the present invention is obviously higher than the silybin bulk drug and physical mixture, see appendix figure 2 .
[0032] Dissolution assay:
[0033] Get an appropriate amount of sample (equivalent to silibinin 35mg), according to the dissolution assay (Chinese Pharmacopoeia 2015 edition four general rules 0931 second method), with 0.5% SDS aqueous solution 900ml as the dissolution medium, the rotating speed is 100r / min, operate according to law, After 10, 15, 20, 30, 45, and 60 minutes, take 5ml o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com