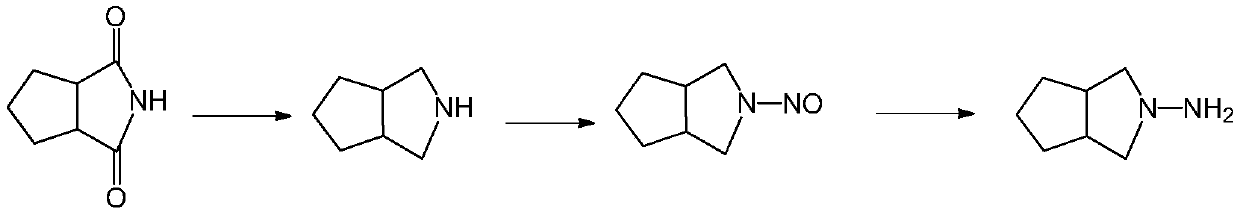
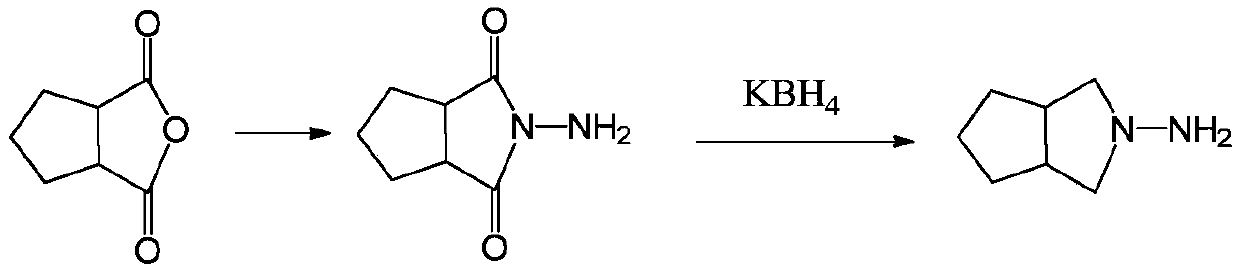
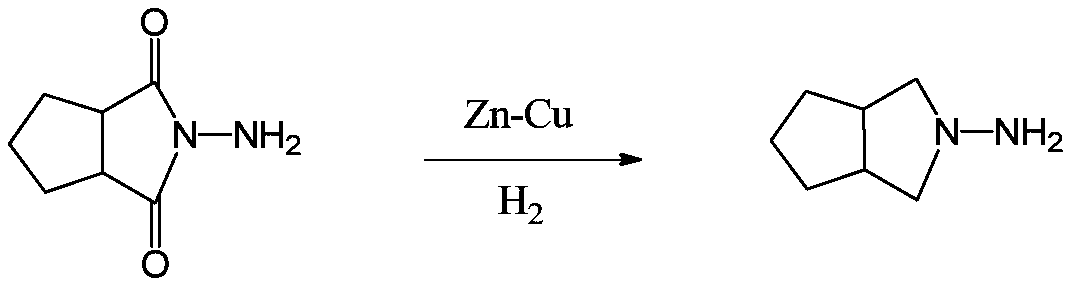Preparation method of gliclazide side chain and preparation method of gliclazide
A technology of gliclazide and side chain, which is applied in the preparation of gliclazide and the new preparation field of side chain of diabetes drug gliclazide, which can solve the problems of difficult industrialization, harsh reaction conditions, and high equipment requirements. Achieve the effects of reduced preparation cost, low hydrogen pressure, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The invention provides a preparation method of gliclazide side chain N-amino-3-azabicyclo[3.3.0]octane (I), using N-aminocyclopentane imide (II) as raw material, A highly active ruthenium carbon catalyst modified with a transition metal atom is used for one-step hydrogenation to obtain a gliclazide side chain (I), and the specific preparation method is as follows:
[0040]
[0041] Dissolving N-aminocyclopentane imide (II) in an acidic aqueous solution, adding a highly active ruthenium-carbon catalyst modified by a transition metal atom, under the condition that the hydrogen pressure is 6-9MPa and the temperature is 90-140°C, Reduction for 16 to 20 hours produces N-amino-3-azabicyclo[3.3.0]octane (I). Then cool down to room temperature, release the pressure, and filter. The filter cake ruthenium carbon catalyst can be applied (recycled) to the next reaction. The filtrate is concentrated under reduced pressure. The acid water is usually recovered after distillation an...
Embodiment 1
[0064] (1) Preparation of molybdenum atom modified ruthenium carbon catalyst
[0065] Weigh 47.26kg of activated carbon powder and disperse it in 200L of deionized water; weigh 6.723kg of ruthenium chloride trihydrate and dissolve it in 100L of deionized water; under stirring conditions, mix the two and absorb for 1 hour; weigh 1.911kg of molybdic acid Ammonium solid ((NH 4 ) 6 Mo 7 o 24 4H 2 (2), be dissolved in 50L deionized water, and gradually add in the above-mentioned gac solution under stirring condition; After stirring and absorbing for 1 hour, add sodium borohydride aqueous solution (1.2kg sodium borohydride is dissolved in 100L deionized water) gradually, react 1 at room temperature Hours later, filter with suction, wash 5 times with deionized water to near neutrality, and dry in an oven at 80° C. for 12 hours to obtain a molybdenum atom-modified ruthenium carbon catalyst, wherein the mass percentage of ruthenium is 5 wt%, and the mass percentage of molybdenum T...
Embodiment 2
[0072] In the 500L autoclave, add N-aminocyclopentane imide 100kg, acetic acid 70L, water 250L, stirring and dissolving, add the molybdenum atom modified ruthenium carbon catalyst (ruthenium content: 5wt% of 5wt%) prepared in embodiment 1 (1), Molybdenum content: 2wt%) 15kg. Under the conditions of controlling the hydrogen pressure to 8 MPa and the temperature to 135° C., the reaction was carried out for 20 hours. Cool down to room temperature, release the pressure, and filter. The filter cake ruthenium carbon catalyst sleeve is used for the next reaction. The filtrate was transferred to a concentration kettle to concentrate under reduced pressure, and the acetic acid water jacket was recovered for the next reaction. Concentrate to obtain about 114kg of the crude gliclazide side chain. Add hydrochloric acid to process, then add toluene-ethanol mixed solvent to recrystallize, centrifuge shakes off material, oven dry, obtain N-amino-3-azabicyclo[3.3.0]octane hydrochloride 97....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com