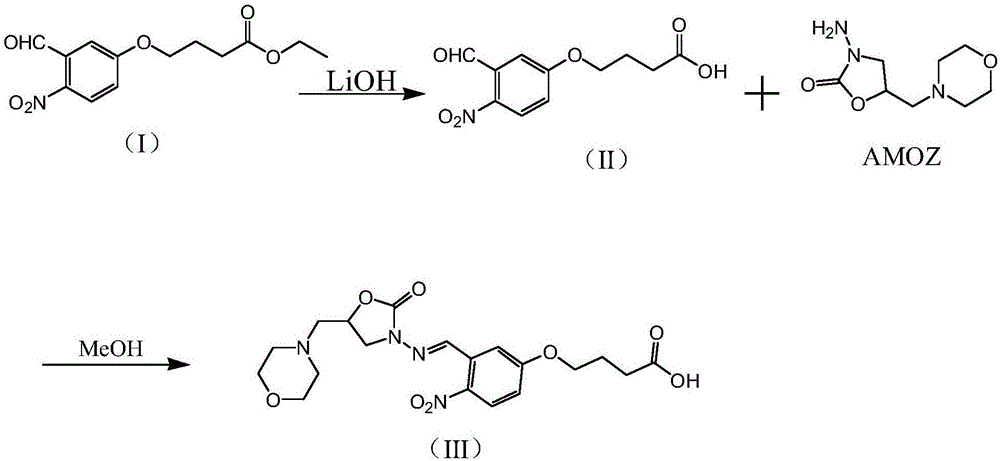
Furaltadone metabolite (AMOZ) derivatization hapten, preparation method of artificial antigen and application of artificial antigen
A technology of furaltadone and artificial antigen, which is applied in the field of immunochemistry, can solve the problem that the AMOZ hapten cannot completely retain the characteristic structure of the competitor, and achieve the effects of enhanced immunogenicity, enhanced structural characteristics, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Such as Figure 1-Figure 5 As shown, the furaltadone metabolite AMOZ derivatized hapten in Example 1 is prepared by the following method, and the steps are as follows:
[0052] (a') Dissolve 2.0 g (ie 7.1 mmol) of compound I in 10 ml of ethanol, adjust the pH value of the ethanol solution of compound I to 10-12 by adding 6 mol / L lithium hydroxide solution, and react at room temperature for 15 ~26h, then add 40ml of purified water, adjust the pH value of the resulting solution to 4~5 with 1M dilute hydrochloric acid, filter, and dry to obtain 1.1g of compound II.
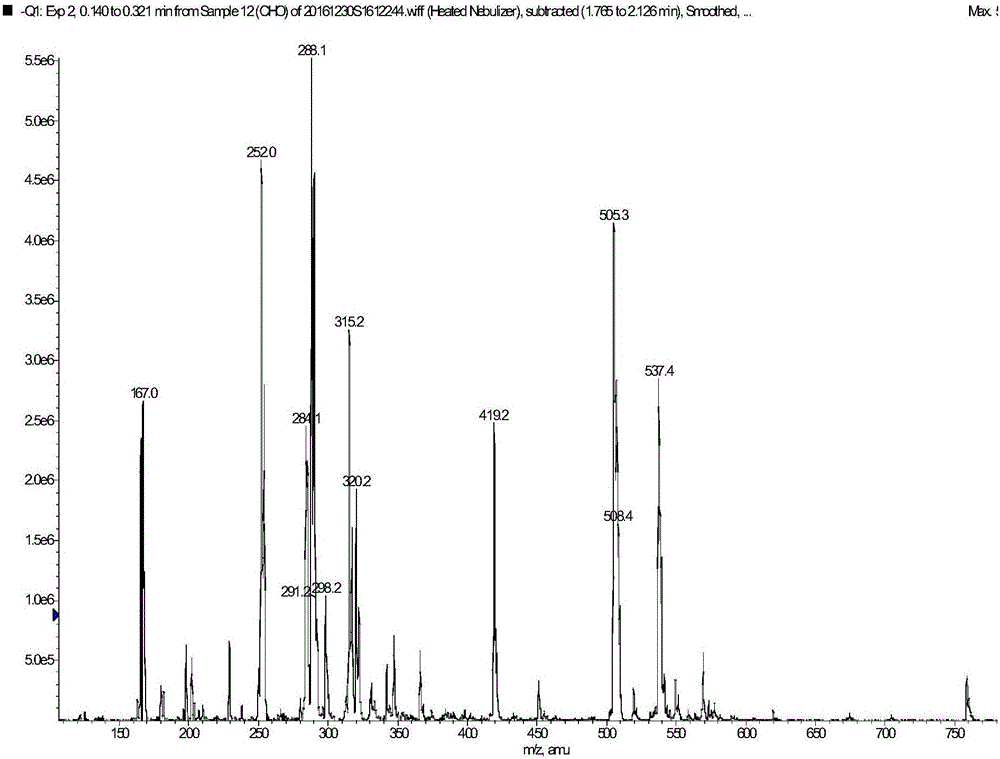
[0053] ESI-MS: 167[M-CH2CH2CH2COOH-1], 252[M-1], 288[M+2H2O-1], 315[2×167-H2O-1], 505[2M-1], 537[2M +MeOH-1];
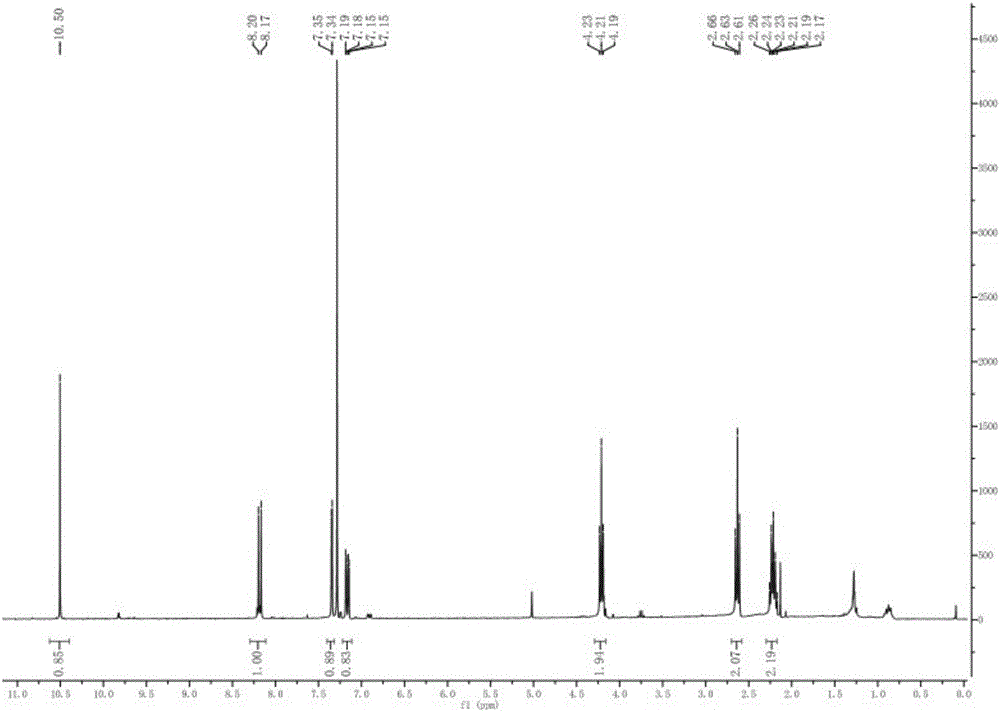
[0054] 1H NMR (600MHz, CDCl 3 , TMS): δ10.50(s, 1H), 8.20(d, 1H), 7.35(d, 1H), 7.15-7.19(dd, 1H), 4.23(t, 2H), 2.66(t, 2H), 2.26 (m, 2H).
[0055] (b') Dissolve 0.5g (ie 2.0mmol) of compound II in 10ml of methanol, add 0.48g (ie 2.4mmol) of furaltadone metabolite AMOZ, react at 60-70°C for 2h, a...
Embodiment 2
[0059] Example 2 is the artificial antigen of the furaltadone metabolite AMOZ. The artificial antigen includes an immunogen and a coating. The difference between the two lies in the types of carrier proteins coupled during the preparation process. For the hapten prepared in Example 1, the carrier protein is bovine serum albumin (BSA); the coating source is the hapten prepared in Example 1, and the carrier protein is ovalbumin (OVA).
[0060] The preparation method of the coating source of the furaltadone metabolite AMOZ of the present embodiment 2, the steps are as follows:
[0061] (1) Take 10.0 mg of the hapten of Example 1, dissolve it in 0.5 ml of dimethylformamide (DMF), stir well, add 8.0 mg of carbodiimide (EDC) and 6.0 mg of N-hydroxysuccinimide Amine (NHS), stirred at room temperature for 4-8h, to obtain hapten activated ester;
[0062] (2) Weigh 30.6mg of ovalbumin (OVA), make it fully dissolved in 4ml of PBS solution with a concentration of 0.01mol / L to form a carr...
Embodiment 3
[0074] The monoclonal antibody of the furaltadone metabolite AMOZ in Example 3 was prepared by the following method, the steps are as follows:
[0075] After emulsifying the immunogenic formula (III)-BSA of Example 2 with an equal volume of Freund's adjuvant, immunize BALB / C mice. The immunization dose for each mouse was 50-100 μg, and the immunization interval was 3 weeks. After 3 times of immunization, the blood of the tail vein of the mice was collected to detect the serum titer. If the antibody titer does not reach 60,000, booster immunization is required. After the antibody titer no longer rises, subcutaneous booster immunization is performed with 100 μg of immunogen, and the splenocytes of the mouse are fused with SP20 cells 5 days later. The fused cells were selected in HAT medium, and after 5 days, the complete medium was replaced with HAT medium for culture. Use ELISA to detect the cell supernatant, and carry out the limited dilution clone culture of the cells in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com