Recombinant attenuated Salmonella enteritidis vaccine
A Salmonella Enteritidis and vaccine technology, applied in the direction of viruses/phages, bacteria, viruses, etc., can solve the problems of lack of and inability to stably express foreign proteins, save labor costs, prevent and control Salmonella infection, and prevent and control IBDV. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, the screening of Salmonella enteritidis attenuated strain
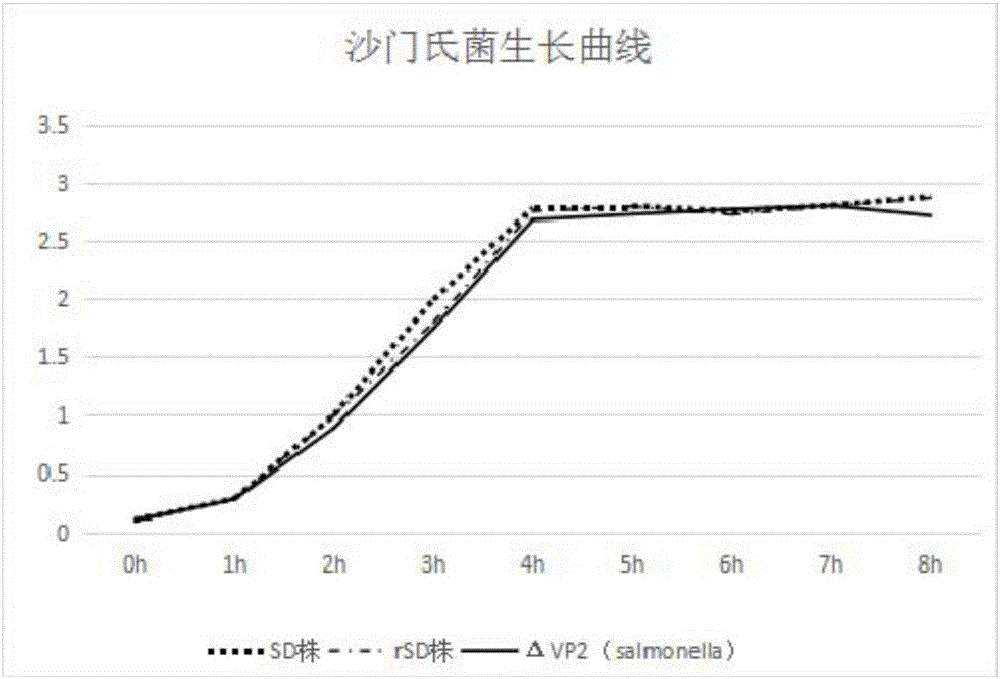
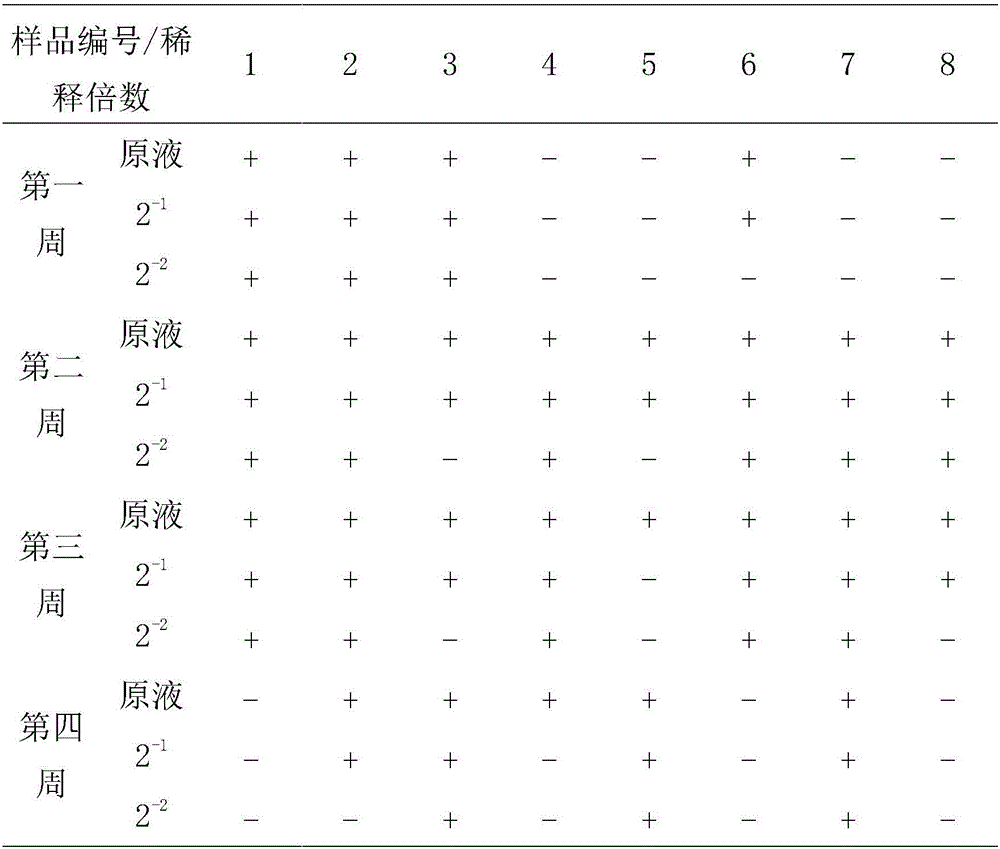
[0018] After conducting biochemical tests, serological tests and sequencing identification on the suspected Salmonella isolated clinically, the applicant identified it as Salmonella Enteritidis and named it SD strain. The recombinant strain rSD of Salmonella enteritidis was obtained after subculture screening. Through the determination of the growth curve, it was found that the growth rate of the bacteria was not affected by the deletion of the gene, and it was determined to be an attenuated strain through the chick challenge test. The rSD strain was used as the parent strain for further research.
[0019] The attenuated Salmonella Enteritidis rSD strain of the present invention was preserved on November 28, 2016 in the General Microbiology Center of the China Microbiological Culture Collection Management Committee, address: No. 3, No. 1, Beichen West Road, Chaoyang District, Beijing, Institute o...
Embodiment 2
[0020] The construction of embodiment 2, VP2 gene transfer vector
[0021] According to the sequence of the Cat gene in the plasmid pkD3 and the multiple cloning restriction site of the cloning vector pBluescript KS (+ / -), the specific primers of the Cat gene were designed using Primer 5.0 software: upstream primer pKD3-II P1:CACGGATCCgtgtaggctggagctgcttc added BamH I Restriction site; downstream primer pKD3-II P2:CAGGAATTCcatatgaatatcctccttag added EcoR I restriction site. The plasmid pkD3 was used as a template for PCR amplification, and the target gene was recovered with a DNA purification and recovery kit. The recovered target gene fragment was subjected to double enzyme digestion reaction with the carrier pBluescript KS (+ / -), followed by nucleic acid electrophoresis. After recovering the target gene with a DNA purification and recovery kit, it was ligated with T4 DNA ligase to obtain a recombinant plasmid. The recombinant plasmid was transformed into Escherichia coli JM...
Embodiment 3
[0023] Embodiment 3, preparation of targeting fragment
[0024] The primers used for homologous recombination consist of two parts, the uppercase 50nt sequence at the 5' end is homologous to the flanking sequence of the target gene, and the lowercase 20nt sequence at the 3' end is identical to the sequence on both sides of the transfer vector cat resistance gene or the target gene source. Upstream primer: VP2-D1: AGTGGACTAACAACATCGGTGATGCCAACACCATCGGCACCCGTCCGGACgtgtaggctggagctgcttc; Downstream primer: VP2-D2: CAGAGCAAAAAACCCCGCGACGCGGGGTTTTTATCAGACGGAAACTTAAttaccttatggcccggat. Using the recombinant plasmid pBKV as a template, the target fragment was amplified by PCR, and the PCR product was identified by 1% agarose gel electrophoresis, the target band was recovered, and the recovered product was digested with Dpn I. After 1% agarose gel electrophoresis, the target band was recovered by cutting the gel, and the DNA concentration was determined.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com