Preparation method of avenanthramide and derivative thereof
A technology for oat alkaloids and derivatives, applied in the field of preparation of oat alkaloids and their derivatives, can solve the problems of high cost, low yield, high reaction temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
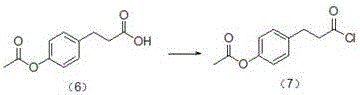
[0027] ① Weigh 180.0g of p-acetoxyphenylpropionic acid and suspend it in 90mL of toluene, quickly drop 400.0g of thionyl chloride into it at room temperature, and after overnight, react at 80°C for 2h and distill until no obvious fraction distills out to obtain brown Yellow liquid - p-acetoxyphenylpropionyl chloride, yield 94.0%.
[0028]
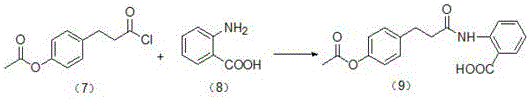
[0029] ②Weigh 100.0g of anthranilic acid and suspend in 1200mL of dichloromethane, under the protection of nitrogen, control the temperature at 10°C, add dropwise 220.0g of p-acetoxyphenylpropionyl chloride obtained in step ①, and drop it in about 2.0h. Then add 120.0g of triethylamine dropwise, control the temperature at 10°C, drop it for about 1h, keep stirring for 0.5h, add 500.0g of cold hydrochloric acid (2.0%), evaporate dichloromethane, filter with suction, wash with water, and dry to obtain brown Yellow solid, yield 81.1%.
[0030]
[0031] ③ Add 600.0 g of sodium hydroxide (15%) to the reaction product obtained in step ②, co...
Embodiment 2
[0034] ①Weigh 160.0g of p-acetoxyphenylacrylic acid and suspend it in 100mL of chloroform, quickly drop 400.0g of thionyl chloride into it at room temperature, stir overnight, react at 80°C for 3.0h, and distill off excess solvent and thionyl chloride , to obtain brownish yellow liquid - p-acetoxy phenylacryloyl chloride, yield 93.0%.
[0035]
[0036] ②Suspend 100.0g of anthranilic acid in 1200mL of dichloromethane, under the protection of nitrogen, control the temperature at 10°C, add dropwise 222.0g of p-acetoxyphenylacryloyl chloride obtained in step ①, and drop it in about 2.5 hours. Then add 150.0g sodium hydroxide (30%) dropwise, control the temperature at 5-10°C, drop it for about 1h, continue stirring for 1.5h, add 500mL cold hydrochloric acid (2%), distill off dichloromethane, suction filter, wash with water , and dried to give a light brown solid with a yield of 91.2%.
[0037]
[0038] ③Add 600.0g sodium hydroxide (15%) to the reaction product in step ②, con...
Embodiment 3
[0041] ①Weigh 180.0g of 2-methoxy-3-acetoxyphenylacrylic acid and suspend it in 100mL of cyclohexane, quickly drop 400.0g of thionyl chloride into it at room temperature, react overnight at 80°C for 2.5h, and distill off Excessive solvent and thionyl chloride give a brownish-yellow liquid, which is set aside.
[0042]
[0043] ②Suspend 120.0g of 2-amino-5-hydroxybenzoic acid in 1500.0g of chloroform, under the protection of nitrogen, control the temperature at 10°C, add dropwise the 3-methoxy-4-acetoxybenzene obtained in step ① 240.0g of acryloyl chloride, dripped for about 2.5 hours, then added dropwise 110.0g of triethylamine, controlled the temperature at 10°C, dropped for about 1 hour, kept stirring for 0.5 hours, added 100.0g of cold hydrochloric acid (10%), distilled under reduced pressure Chloromethane, suction filtered, washed with water, and suction filtered to obtain a light yellow solid with a yield of 91.8%.
[0044]
[0045] ③Add 310.0g sodium hydroxide (30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com