AMP (antimicrobial peptide) fusion protein as well as preparation method and application thereof
A fusion protein and antimicrobial peptide technology, which is applied in the direction of peptide/protein components, antibacterial drugs, antifungal agents, etc., can solve the problems of unsuitability for large-scale production, many technical bottlenecks of antimicrobial peptides, and high synthesis costs, etc., to ensure fusion Protein activity, guaranteed purification and refolding, and high antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
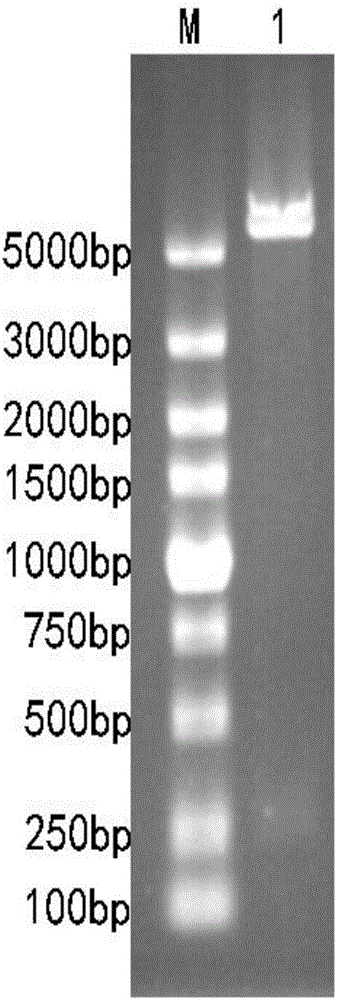
[0037] Embodiment 1: Construction of recombinant expression vector
[0038] 1. Design and synthesis of the target fragment
[0039] Obtain cecropin nucleotide sequence (as shown in SEQ ID NO.5) by NCBI database, find shark liver peptide orf-86 gene (as shown in SEQ ID NO.6) in the shark liver small peptide cDNA database of this laboratory , orf-86 shark liver peptide is a small peptide translated from the shark liver peptide orf-86 gene with an isoelectric point of 11 that may have potential biological activity screened from the regenerated liver cDNA library of the striped bamboo shark. Add 3 tandem fragments (Gly+Ser+Ala) coding genes (as shown in SEQ ID NO.7) in the middle of the acid sequence to form shark liver peptide orf-86+3GSA coding fragment+cecropin fusion gene (hereinafter referred to as orf- 86 fusion gene, as shown in SEQ ID NO.4), and a BamH I restriction site is added to its 5' end, and an Xho I restriction site is added to the 3' end, which is synthesized by ...
Embodiment 2
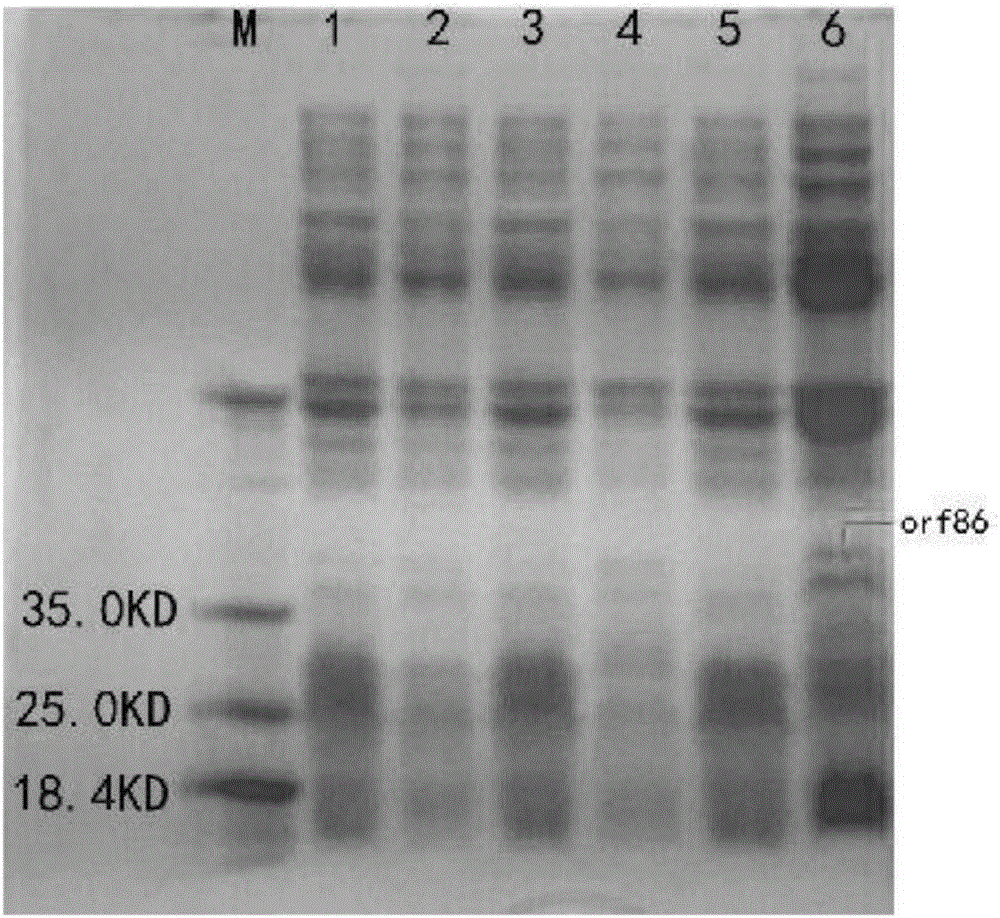
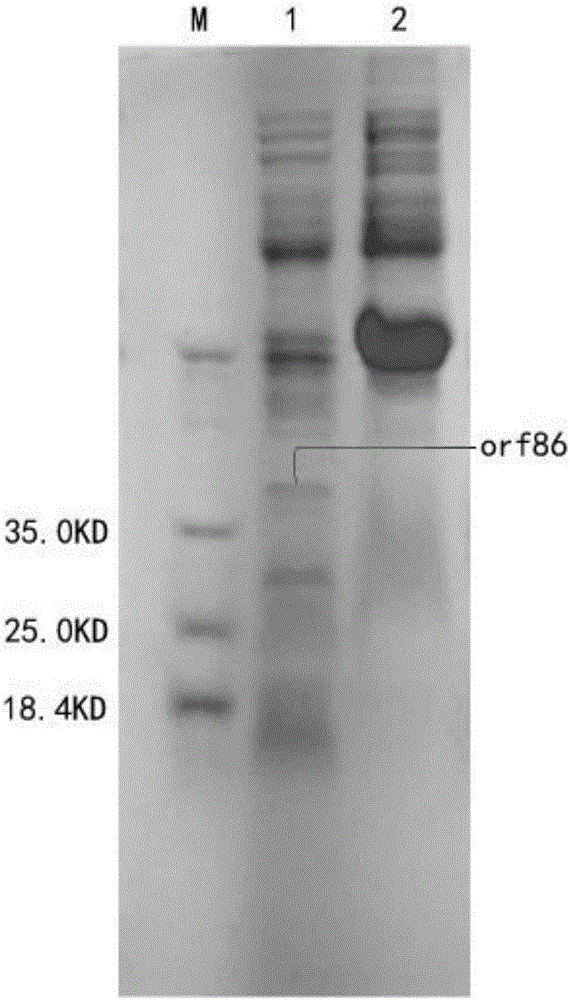
[0046] Example 2: Induced expression and concentration of fusion proteins
[0047] 1. Induced expression of recombinant fusion protein
[0048] After the sequencing was correct, the plasmid was extracted, and the recombinant plasmid was transformed into BL21(DE3) Escherichia coli for induced expression.
[0049] The two successfully constructed engineering bacteria were inoculated in 100mL LB culture medium, and cultured in a constant temperature shaker at 37°C until A 600 When the value is about 0.6, add IPTG to a final concentration of 1 mmol / L, induce at 16°C for 20 hours, collect the bacteria by centrifugation at 12,000 rpm, wash twice with PBS, and ultrasonically break the induced recombinant bacteria for 30 minutes, sonicate for 3 seconds, stop for 3 seconds, and then Centrifuge at 12000rpm for 25min, take the supernatant and precipitate respectively, and detect the existence form of the expression product by SDS-PAGE electrophoresis, the results are as follows figure...
Embodiment 3
[0055] Embodiment 3: Oxford cup method detects antibacterial activity
[0056] Detection of antibacterial activity of recombinant protein orf-86
[0057] Take the agar powder and solid medium sterilized at 121°C for 20 minutes into the ultra-clean bench, first pour a thin layer of agar powder on the glass petri dish, and then separate the activated TG1 Escherichia coli and Bacillus subtilis Add it to the solid medium whose temperature has dropped to about 50°C, and pour the solid medium containing the bacteria solution after the agar powder solidifies. After the plate is solidified, put the sterilized Oxford cup that has been burned by the flame of an alcohol lamp on the plate to form a sample hole, and add 200 μL of Amp (1mg / mL), ddH2O, orf86 to the Oxford cup, and Amp is Positive control, ddH2O as negative control. After culturing overnight at 37°C, observe the size of the inhibition zone to judge the antibacterial activity of the sample.
[0058] The results showed that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com