Eight-arm heteroarm star-shaped polymer and preparation method thereof
A technology of star polymers and heteroarms, which is applied in the field of preparation of eight-arm heteroarm star polymers, can solve the problem of poor controllability of molecular weight and molecular weight distribution of polymer arms, consistent initiation efficiency of initiation points, and multifunctional initiators. Difficulties and other problems, to achieve the effects of good molecular weight and molecular weight distribution control, mild reaction conditions, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Preparation of eight-arm hybrid-arm star polymer (7PS-POSS-PCL)
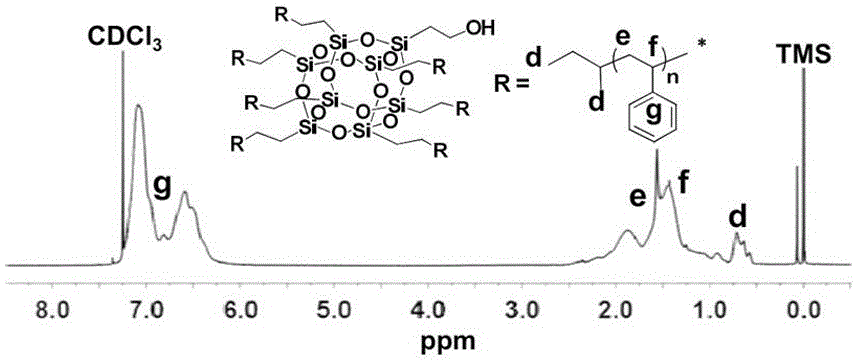
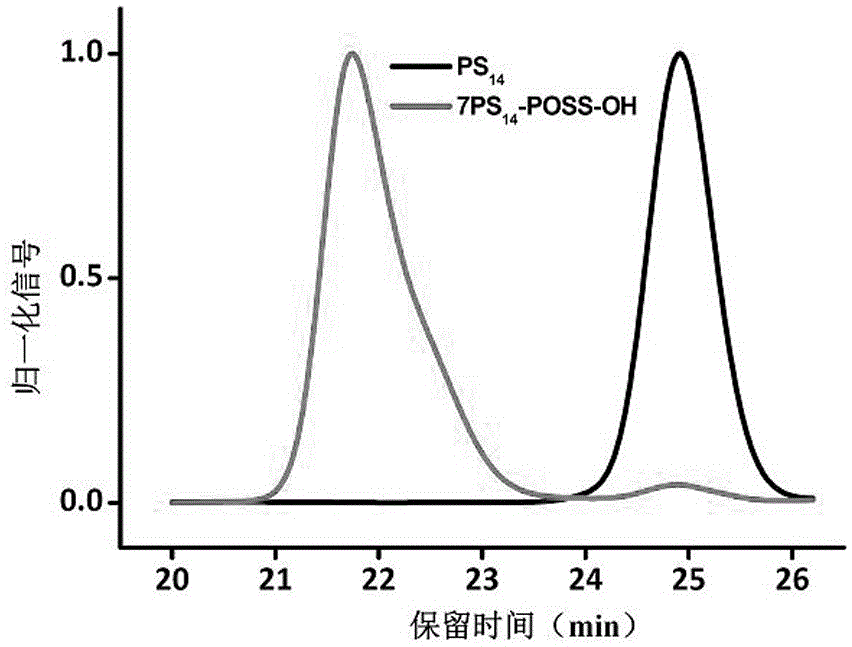
[0080] (1) Preparation of seven-arm star polystyrene (7PS-POSS-OH) containing a single hydroxyl group
[0081] Using sec-butyllithium (sec-BuLi, 3.8 mL, 4.96 mmol) as the initiator and benzene (50 mL) as the solvent, the living anionic polymerization of styrene (7.8 mL, 68.1 mmol) was initiated at 30 °C, reaction 8 After 1 hour, a small amount of active chain solution was taken and terminated with methanol, and the remaining active chain continued to undergo addition reaction directly with VPOSS-OH (389.3 mg, 0.6 mmol) under vacuum conditions, and was terminated with methanol after 1 hour of reaction.
[0082] After the reaction, the product was subjected to rotary evaporation to remove the solvent, and precipitated in methanol, filtered and concentrated to obtain a white solid powder, which was dried in a vacuum oven at room temperature for 24 hours to obtain the crude product of 7PS-POSS-OH, ...
Embodiment 2
[0094] Example 2: Preparation of eight-arm hybrid-arm star polymer (7PS-POSS-PEG)
[0095] (1) Preparation of alkyne-terminated polyethylene glycol (Propargyl-PEG)
[0096] A certain amount of mPEG-OH (2.00 g, 1 mmol) and 40 mL of anhydrous toluene were added to a 100 mL branched flask, and water was removed azeotropically. After the end, the purified mPEG-OH was dissolved in anhydrous THF under an inert gas atmosphere, KH (0.24 g, 6 mmol) was added slowly, and the reaction was stirred for 1 h in an oil bath at 25 °C.
[0097] Add 3-bromopropyne (1.19 g, 10 mmol, 80% in Toluene) into the constant pressure dropping funnel with a syringe, and dilute with about 5 mL of THF. Then it was added dropwise into the branch bottle, and reacted in an oil bath at 45°C for 20 h after the dropwise addition was completed. After filtration and concentration, add 40 mL of CH 2 Cl 2 , with saturated NaHCO 3 The solution was extracted three times, and the organic phase was collected and wash...
Embodiment 3
[0102] Example 3: Preparation of eight-arm hybrid arm star polymer (7PS-POSS-PDMA)
[0103] (1) Preparation of seven-arm star polystyrene (7PS-POSS-OH) containing a single hydroxyl group
[0104] Using sec-butyllithium (sec-BuLi, 3.0 mL, 3.92 mmol) as the initiator and benzene (40 mL) as the solvent, the living anionic polymerization of styrene (8.16 mL, 78.4 mmol) was initiated at 30 °C for 10 After 1 hour, a small amount of active chain solution was taken and terminated with methanol, and the remaining active chain continued to undergo addition reaction directly with VPOSS-OH (259.5 mg, 0.4 mmol) under vacuum conditions, and was terminated with methanol after 1 hour of reaction.
[0105] After the reaction, the product was subjected to rotary evaporation to remove the solvent, and precipitated in methanol, filtered and concentrated to obtain a white solid powder, which was dried in a vacuum oven at room temperature for 24 hours to obtain the crude product of 7PS-POSS-OH, Us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com