Benzylidene indandione compound and preparation thereof and application in specific imaging of lipid droplet
A technology of benzylidene indanedione and compound is applied in the application field of lipid droplet-specific fluorescence imaging, which can solve the problems of limited application, aggregation-induced quenching, high cost, etc., and achieves large Stokes shift and low cytotoxicity. , easy-to-prepare effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Benzylidene indenedione compound IND-TPA: 2-(4-(diphenylamino)benzylidene)-1H-indene-1,3(2H)-dione (R 1 = Hydrogen, R 2 =R 3 =Phenyl) The structural formula is as follows:
[0053]
[0054] The preparation method of the above-mentioned benzylidene indenedione compound includes the following steps:
[0055] 4-Diphenylaminobenzaldehyde (273mg, 1.0mmol), 1,3-indenedione (146mg, 1.0mmol) and morpholine (85mg, 1.0mmol) were dissolved in 15mL of ethanol, followed by refluxing under nitrogen protection (Reaction at 78°C for 3 hours); after the reaction is over, return to room temperature, filter and vacuum dry to obtain the red solid product 2-(4-(diphenylamino)benzylidene)-1H-indene-1,3( 2H)-Diketone (285 mg, 71% yield). The relevant structural characterization data are as follows:
[0056] 1 H NMR(DMSO-d 6 ,500MHz): δ8.49(d,J=9.0Hz,2H),7.93-7.89(m,4H),7.71(s,1H),7.48-7.45(m,4H),7.30-7.26(m,6H) ), 6.85(d,J=9.0Hz,2H); 13 C NMR(CD 2 Cl 2 ,125MHz):191.3,189.6,152.7,146.6,145.8,142.4...
Embodiment 2
[0058] Characterization of the photophysical properties of the compound IND-TPA prepared in Example 1:
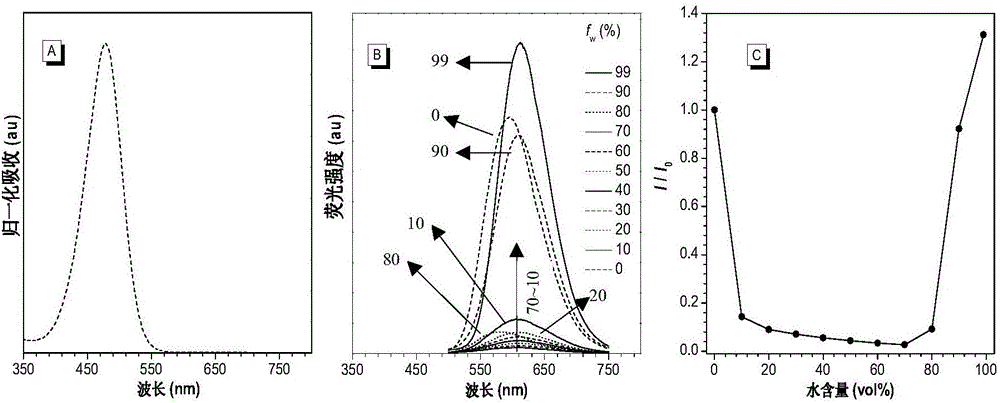
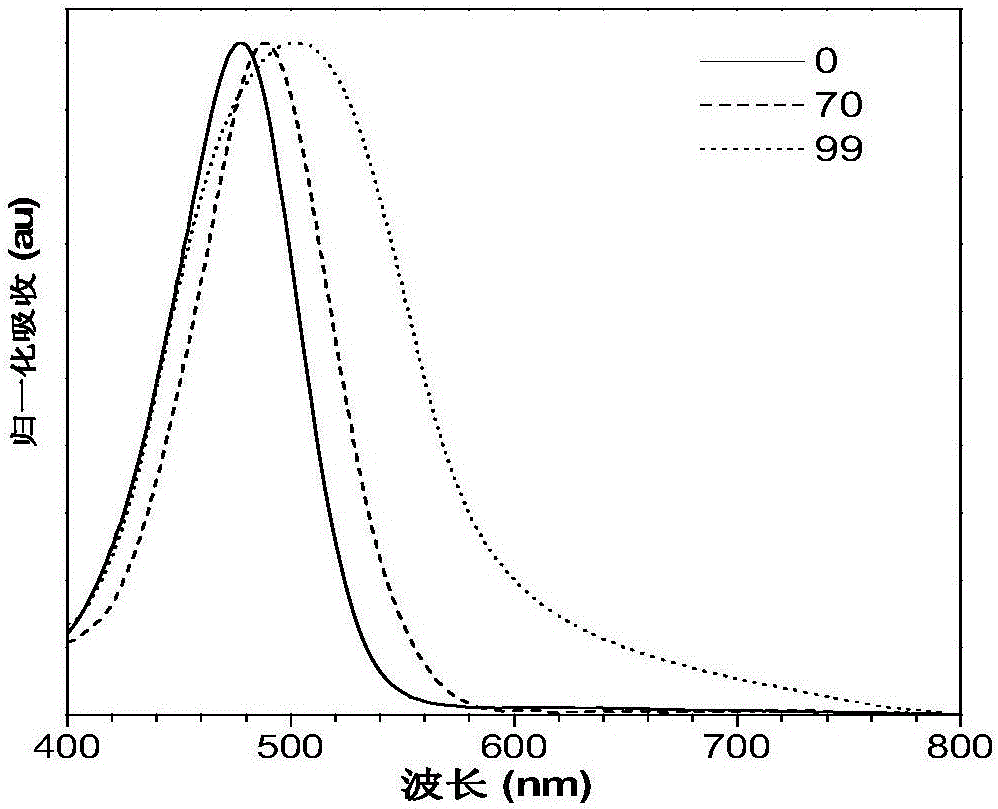
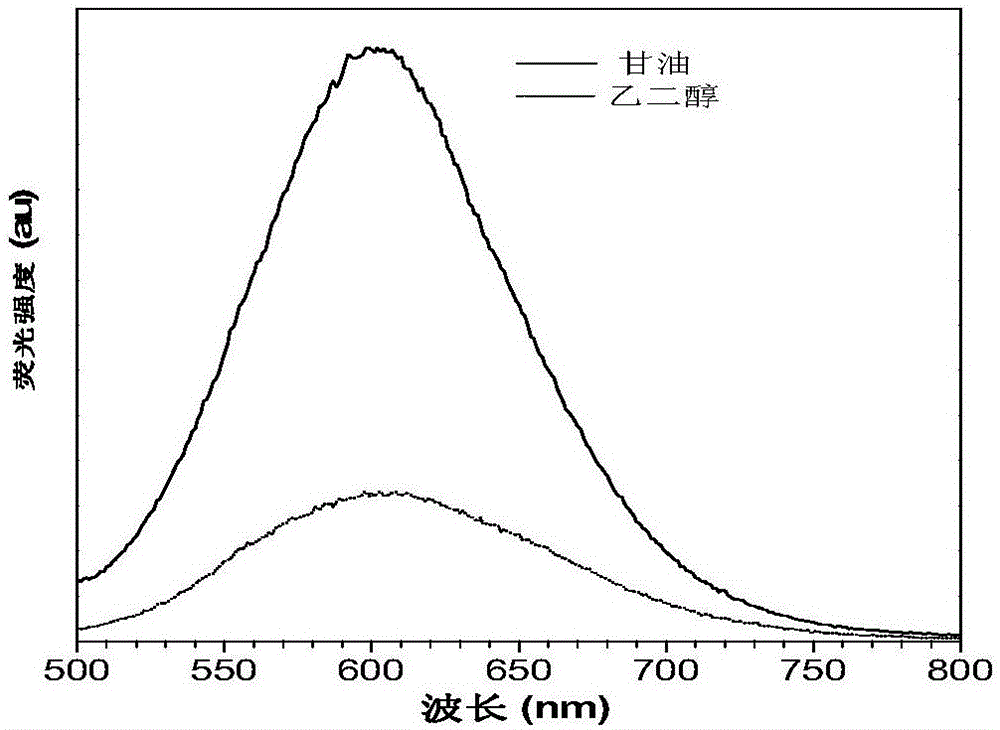
[0059] Combine tetrahydrofuran and water in different volume ratios (tetrahydrofuran: water = 100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 1:99) to form the water content (f w ) Different mixtures, dissolve the compound IND-TPA into these mixtures to make the concentration of the compound 10 -5 mol·L -1 , And then detect the fluorescence emission spectrum and the ultraviolet absorption spectrum in tetrahydrofuran, the result is figure 1 . figure 1 The UV absorption and fluorescence emission spectra of the compound IND-TPA prepared in Example 1; (A) the normalized UV absorption spectrum of the compound IND-TPA in tetrahydrofuran; (B) the compound IND-TPA in tetrahydrofuran and water Fluorescence emission spectra of increasing water content in the mixed solution (10 - 5 mol·L -1 ); (C) is the ratio of the maximum fluorescence emission intensity of compoun...
Embodiment 3
[0067] Cytotoxicity test of the compound IND-TPA prepared in Example 1:
[0068] The experimental results of the cytotoxicity of the compound IND-TPA against lung cancer cells HCC827 and A549 are as follows Figure 5 Shown. This result shows that the compound IND-TPA has almost no cytotoxicity at different concentrations (5, 10, 20, 40, 60, 80, 100 μM).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com